11) HomemadeSolutionof NaODin D2O: Applicationsin the FieldofStilbene-d1Synthesis
Keisuke Imai, Naohito Tomita, Hiroyoshi Fujioka, Mako Kamiya, Riku Ogasahara, Kazuho Ban, Hyoga Shimizu, Takayoshi Ishimoto, Hironao Sajiki, Shuji Akai, Yoshinari Sawama
Asian J. Org., Chem. 2023, e202200690.Cited as a Front Cover
DOI: 10.1002/ajoc.202200690
Selected as Top Downloaded Article between 1st January 2022-31st December 2022, up to 12 months after publication
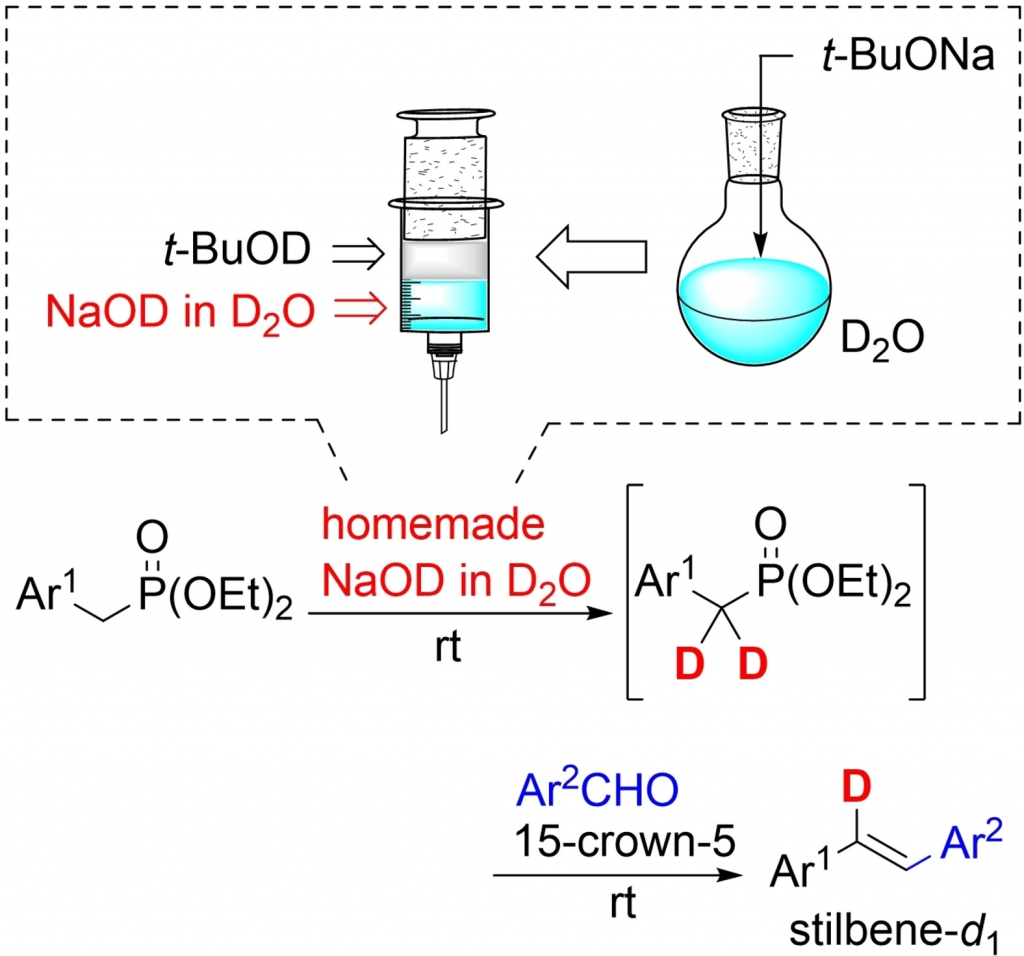

Abstoract: The development of deuterium-incorporation method is eagerly desired. A solution of sodium deuteroxide in deuterium oxide (NaOD in D2O) was simply prepared by mixing D2O and t-BuONa. Subsequently, the t-BuOD layer, generated under conditions of the salting-out effect, was separate. The purity (volume of the contaminated t-BuOD as side-product) of the homemade NaOD in D2O was evaluated by 1H NMR and Raman analyses. NaOD in D2O was used for the one-pot synthesis of mono-deuterated trans-stilbene (stilbene-d1) in the aqueous Horner-Wadsworth-Emmons reaction. The present method can be widely applied to synthesize the various mono-deuterated stilbene derivatives with high deuterium content. Additionally, the obtained mono-deuterated stilbene derivatives could be successfully converted to various deuterated compounds.

10) Oxidative Two-way Regiocontrolled Coupling of 3-Methoxycarbonylcatechol and Indoles to Arylindoles
Yoshinari Sawama, Shoko Kuwata, Miyu Mae, Taro Udagawa, Shuji Akai, Hironao Sajiki
Chem. Commun., 2022, 58, 12935–12938. Cited as a Back Cover
DOI: 10.1039/D2CC04843D
インドールの重水素化
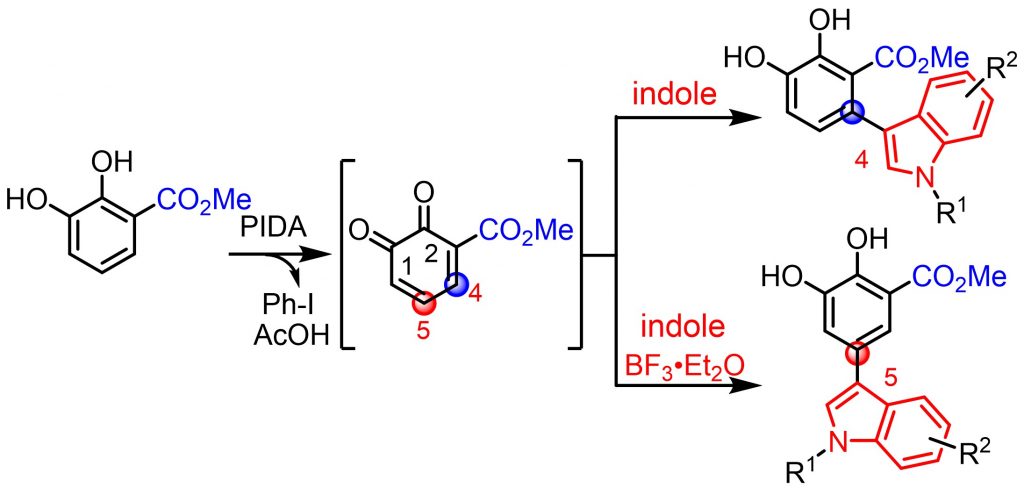

Abstract: 3-Methoxycarbonylcathechol effectively underwent the two-way regiocontrolled coupling with indoles via an ortho-benzoquinone intermediate, resulted by phenyliodine(III) diacetate oxidation, to generate 4-adducts or 5-adducts with or without BF3•Et2O in a one-pot manner. DFT calculations confirmed the obtained regioselectivities.BF3•Et2O. DFT calculations confirmed the obtained regioselectivities.

9) Cellulose nanofibers of oil palm biomass in alginate-based membranes for water-ethanol mixture separation
Novitri Hastuti, Hendrik Setiawan, Kyohei Kanomata, Takuya Kitaoka
Cellulose Chemistry and Technology, 2022, 56, 737-747.
DOI: 10.35812/CelluloseChemTechnol.2022.56.65
Abstract: TEMPO-oxidized cellulose nanofibers (TOCNs) from waste of oil palm empty fruit bunches (OPEFB) were integrated into an alginate matrix to increase the capacity of the alginate membrane for water-ethanol separation. The membrane composed of the alginate matrix and TOCNs was characterized in terms of its morphological, physical-mechanical
properties and performance in the separation of water-ethanol suspensions, with ethanol concentrations in the suspension of 10% and 20%. Other alginate membranes integrated with commercial TOCNs from wood were also prepared and tested for comparison. The results showed that the addition of TOCNs (made from wood and OPEFB waste) to the alginate matrix improved the water adsorption capacity of the membrane. The water adsorption capacity of the alginate membranes with wood-derived TOCNs, OPEFB-derived TOCNs and alginate only was 78%, 87% and 66%, respectively. The flux capacity of the alginate membrane, integrated with OPEFB-derived TOCNs, was higher than that of the alginate membrane alone, but lower than that of the alginate membrane integrated with wood-derived TOCNs. This study showed the utilization of nanocellulose from palm oil biomass waste can be considered to improve the physical-mechanical properties of alginate-based membranes used for various applications, including filtration.

8) Enantiodivergent Chemoenzymatic Dynamic Kinetic Resolution: Conversion of Racemic Propargyl Alcohols into Both Enantiomers
Satoshi Horino, Tomoya Nishio, Shinji Kawanishi, Shinya Oki, Koichi Nishihara, Takashi Ikawa, Kyohei Kanomata, Karla Wagner, Harald Gröger, Shuji Akai
Chem. Eur. J. 2022, 28, e202202437. Cited in: Hot Topic: Mesoporous Materials, Cited as a cover art
DOI: 10.1002/chem.202202437
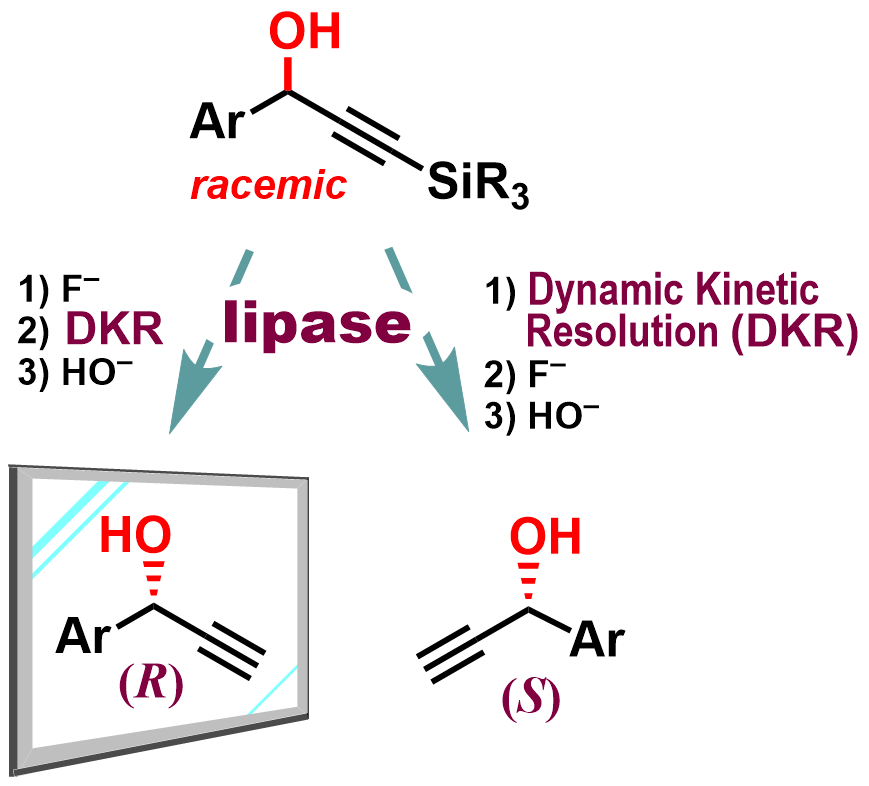

Abstract: Natural lipases typically recognize enantiomers of alcohols based on size differences of substituents near the carbinol moiety and selectively react with (R)-enantiomers of secondary alcohols. Therefore, lipase-catalyzed dynamic kinetic resolution (DKR) of racemic secondary alcohols produces only (R)-enantiomers. We report herein a method for obtaining (S)-enantiomers by DKR of secondary 3-(trialkylsilyl)propargyl alcohols using a well-known (R)-selective Pseudomonas fluorescens lipase in combination with a racemization catalyst VMPS4, in which the silyl group reverses the size relationship of substituents near the carbinol moiety. Since we have already reported (R)-selective DKR of the corresponding propargyl alcohols without substituents on the ethynyl terminal carbon, the presence of an easily removable silyl group has enabled us to produce both enantiomers of propargyl alcohols in high chemical yields and with high enantiomeric excess. In addition, the immobilization of the lipase on Celite was found to be important for achieving the high efficiency of the DKR.

7) 重水素置換低分子化合物の創薬利用と効率的合成
澤間 善成
細胞, 2022, 54, 43-45.


6) 重水素化体の合成と重水素学(Deut-Switch)としての歩み
澤間 善成
生産と技術 2022. 74, 55-58.


5) Aryl Boronic Esters Are Stable on Silica Gel and Reactive under Suzuki−Miyaura Coupling Conditions
Naoki Oka, Tsuyoshi Yamada, Hironao Sajiki, Shuji Akai, and Takashi Ikawa
Org. Lett. 2022, 24, 3510−3514.
DOI: 10.1021/acs.orglett.2c01174. Cited as a cover art
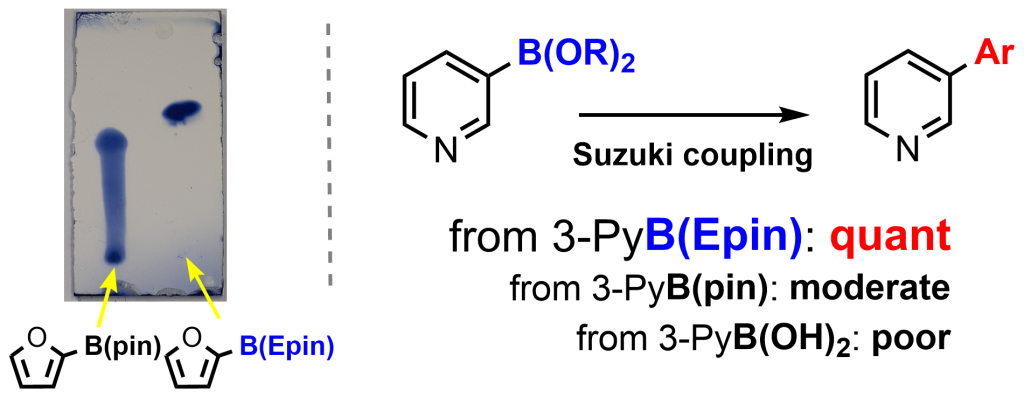

Abstract: A wide range of aryl boronic 1,1,2,2-tetraethylethylene glycol esters [ArB(Epin)s] were readily synthesized. Purifying aryl boronic esters by conventional silica gel chromatography is generally challenging; however, these introduced derivatives are easily purified on silica gel and isolated in excellent yields. We subjected the purified ArB(Epin) to Suzuki−Miyaura couplings, which provided higher yields of the desired biaryl products than those obtained using the corresponding aryl boronic acids or pinacol esters.

4) Metal-free C(aryl)–P bond Ceavage: Experimental and Computational Studies of the Michael Aaddition/Aryl Migration of Triarylphosphines to Alkynyl Esters
Makoto Sako, Kyohei Kanomata, Mohamed S. H. Salem, Tomohiro Furukawa, Hiroaki Sasai and Shinobu Takizawa
Org. Chem. Front. 2022, 9, 2187-2192.
DOI: 10.1039/D2QO00028H
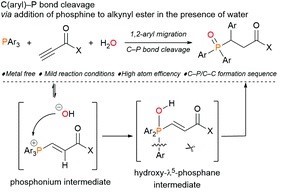
Abstract: The nucleophilic addition and aryl migration of triarylphosphines to alkynyl esters in the presence of water results in the formation of 3-(diarylphosphoryl)-3-aryl propanoic acid derivatives through a metal-free C(aryl)–P bond cleavage process. Experimental and computational investigations of the mechanism indicate that the rapid formation of hydroxy-λ5-phosphane as a key intermediate plays a crucial role in smooth C(aryl)–P bond cleavage.

3) Lipase-Catalyzed Kinetic Resolution of C1-Symmetric Heterocyclic Biaryls
Kengo Kasama, Yuya Hinami, Karin Mizuno, Satoshi Horino, Tomoya Nishio, Chiharu Yuki, Kyohei Kanomata, Gamal A. I. Moustafa, Harald Gröger, and Shuji Akai
Chem. Pharm. Bull. 2022, 70, 391–399.
DOI: 10.1248/cpb.c22-00021. Highlighted Paper selected by Editor-in-Chief
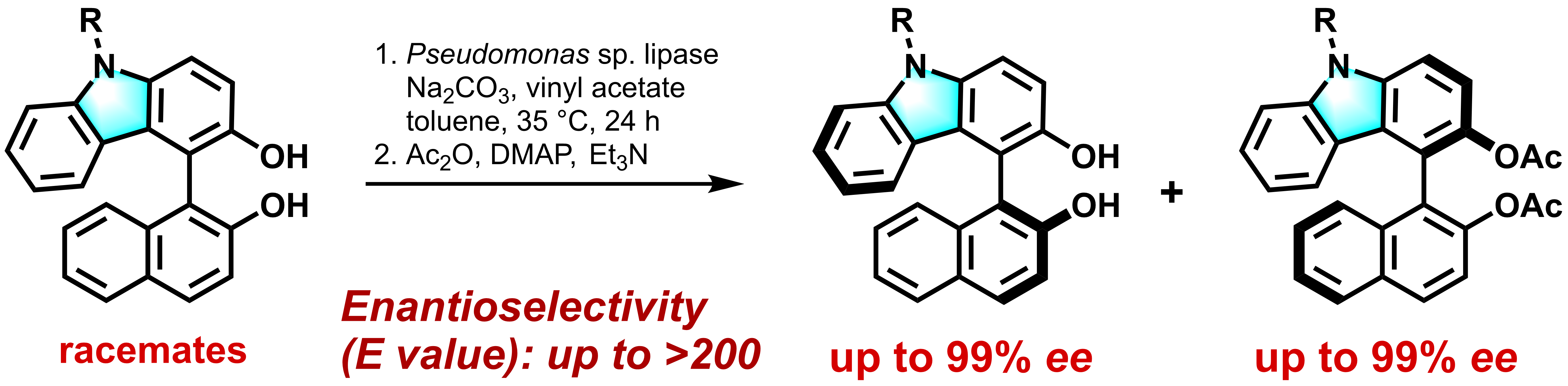
Abstract: The highly enantioselective lipase-catalyzed kinetic resolution (KR) of racemic C1-symmetric biaryl compounds including heterocyclic moieties, such as carbazole and dibenzofuran, has been achieved for the first time. This enzymatic esterification was accelerated by the addition of disodium carbonate while maintaining its high enantioselectivities, and was particularly effective for biaryls having N-substituted carbazole moieties. Furthermore, mesoporous silica-supported oxovanadium-catalyzed cross-dehydrogenative coupling of 3-hydroxycarbazole and 2-naphthol was followed by the lipase-catalyzed KR in one-pot to synthesize the optically active heterocyclic biaryl compounds with high optical purity.

2) Platinum on carbon-catalysed site-selective H–D exchange reaction of allylic alcohols using alkyl amines as a hydrogen source
Park, K., Oka, N., Sawama, Y., Ikawa, T., Yamada, T., Sajiki, H.
Org. Chem. Front., 2022,9, 1986-1991
DOI: 10.1039/D2QO00177B
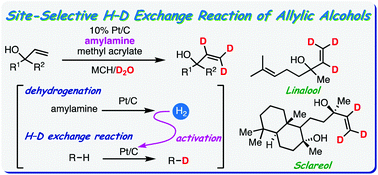
Abstract: We developed a platinum on carbon-catalysed deuteration reaction of tert-allylic alcohols using deuterium oxide as a deuterium source. Amylamine was dehydrogenated by platinum on carbon to generate an appropriate amount of hydrogen gas, which efficiently promoted the H–D exchange reaction. Various allylic alcohols were site-selectively deuterated with good to excellent deuterium contents without hydrogenation of the alkene functionality. The obtained deuterium-labelled allylic alcohols were further transformed into various deuterium-labelled building blocks without degradation of the original deuterium content.

1) 重水素標識機能性材料の網羅的合成を目指して
Exhaustive Syntheses of Deuterium-labelled Compounds
澤間 善成 Yoshinari Sawama
YAKUGAKU ZASSHI, 2022, 142, 2, 139-144.
DOI:10.1248/yakushi.21-00173-2
Deuterium (2H, D) is a stable isotope of hydrogen (1H). Deuterium-incorporated (labelled) compounds are widely utilized in various scientific fields such as mechanistic studies of organic reactions, elucidation of drug metabolism, application as tracers for microanalysis. Recently, development of heavy drugs and molecular imaging using techniques such as neutron scattering and Raman spectroscopy are spotlighted. We have developed various deuterium-incorporated compounds using D2O as an inexpensive deuterium source to construct novel functional materials. The use of platinum group metals on carbon as catalysts could result in the multi-deuteration of compounds in the mixed solvents of 2-propanol and D2O, and site-selectively deuterated compounds can be synthesized by organocatalytic methods. In this review, the latter deuteration methods using organocatalysts and their applications are summarized. Terminal alkynes smoothly underwent deuterium incorporation by using triethylamine as an organic base or a solid resin possessing the tertiary amine moiety in the same molecule to give mono-deuterated alkynes. These compounds were partially reduced over our prepared specific palladium catalyst under atmospheric D2 gas to produce tri-deuterated alkenes. Achiral or chiral di-deuterated β-nitro alcohols were also prepared by the organic-base-catalyzed deuteration of nitromethane, followed by nitroaldol reactions in a one pot manner. The mono-deuteration of aromatic aldehyde could be effectively catalyzed by N-heterocyclic carbene. Furthermore, the α-deuteration of aliphatic aldehydes using a basic resin catalyst and the subsequent Knoevenagel condensation with malononitrile could provide γ-deuterium-incorporated α,β-unsaturated nitrile derivatives. The deuterated compounds thus obtained can be important synthetic precursors to construct the deuterium-ncorporated target functional materials.