15) Synthesis of Fluorinated Aromatic Compounds by One-pot Benzyne Generation/Nucleophilic Fluorinatione
Takashi Ikawa, Shigeaki Masuda, Tsuyoshi Nishiyama, Akira Takagi, Shuji Akai, Aust. J. Chem., 67, 475-480 (2013), DOI:10.1071/CH13548
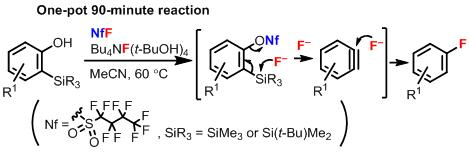
Abstract: The fluorination of substituted benzenes using fluoride ions under mild reaction conditions has been one of the most important challenges for the synthesis of biologically active fluorinated aromatic compounds; however, only a few synthetically useful methods are known. In this paper, we report that the nucleophilic fluorination of benzynes, generated from either 2-(trialkylsilyl)phenyl nonafluorobutanesulfonates or 2-(trialkylsilyl)phenols, meets this challenge. In particular, the fluorination starting from 2-(trialkylsilyl)phenols for fabricating aryl fluorides involves one-pot three sequential reactions: the nonaflylation of phenols, benzyne generation, and nucleophilic fluorination of the benzynes. The regioselectivities of these reactions are controlled by the substituents at the C3-position of the benzynes.
14) ortho-Selective nucleophilic addition of amines to 3-borylbenzynes: Synthesis of multisubstituted anilines by triple role of boryl group
Akira Takagi, Takashi Ikawa, Kozumo Saito, Shigeaki Masuda, Toyohiro Ito, Shuji Akai, Org. Biomol. Chem., 11, 8145–8150 (2013), DOI:10.1039/c3ob41787e
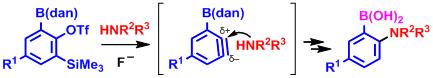
Abstract: Nucleophilic addition of amines to 3-[(dan)boryl]benzynes (dan = 1,8-diaminonaphthalene) generated by a fluoride ion proceeded with high ortho-selectivity to give 2-borylaniline derivatives, under conditions that are tolerant to various functional groups. The (dan)boryl group of the adduct was hydrolyzed into a boronic acid under acidic conditions, which could further serve for various C–C, C–O, C–N, and C–H bond-formation reactions. The overall process provides a promising entry for preparing multisubstituted aniline derivatives.
13) Efficient Intramolecular Cyclizations of Phenoxyethynyl Diols into Multisubstituted α,β-Unsaturated Lactones
Masahiro Egi, Yuya Ota, Yuka Nishimura, Kaori Shimizu, Kenji Azechi, Shuji Akai, Org. Lett., 15, 4150–4153 (2013), DOI:10.1021/ol401824v
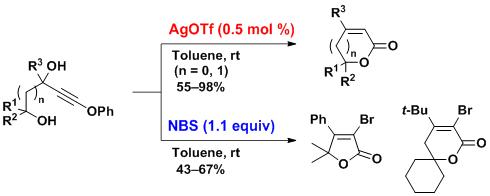
Abstract: AgOTf-catalyzed intramolecular cyclization of phenoxyethynyl diols proceeded under mild conditions to afford the multisubstituted α,β-unsaturated-γ-lactones in 55-98% yields. This method was also applicable to the synthesis of α,β-unsaturated-δ-lactones. A similar cyclization proceeded when AgOTf was replaced with a stoichiometric amount of N-bromosuccinimide to furnish the α-bromo-substituted α,β-unsaturated lactones.
12) Dress-up chiral columns for the enantioseparation of amino acids based on fluorous separation
Kenichiro Todoroki, Yasuhiro Ishii, Koji Toyoda, Takashi Ikawa, Jun Zhe Min, Koichi Inoue, Shuji Akai, Toshimasa Toyo’oka, Anal. Bioanal. Chem., 405, 8121–8129 (2013), DOI:10.1007/s00216-013-7207-4
Abstract: In this paper, we report a new type of chiral high-performance liquid chromatography (HPLC) column–a so-called dress-up chiral column–featuring a chiral stationary phase adsorbed reversibly in a commercial fluorous HPLC column through fluorous interactions. We synthesized perfluroalkylated proline derivatives as chiral stationary phase compounds and then adsorbed them reversibly in the fluorous HPLC column through the pumping of their solutions. By using this dress-up chiral column and fluorophobic elution of an aqueous copper(II) sulfate/MeOH mixture, we could enantioseparate seven racemic amino acids within 40 min. When we washed the dress-up chiral column with fluorophilic tetrahydrofuran or MeOH, the adsorbed chiral stationary phase compounds desorbed from the column, completely destroying its enantioseparation ability. The relative standard deviation of the retention times, the number of theoretical plates, and the resolution for each of four preparations of the dress-up columns were all less than or equal to 9.53% in 20-times repeated analysis, and were all less than or equal to 18.7% in four different preparations, respectively.
11) Takehiko Yoshimitsu, Stereoselective Synthesis of Drugs and Natural Products; Chapter 43:
V. Andrushko, N. Andrushko Eds.,Wiley-Blackwell, Stereoselective synthesis of halogenated natural products (2013), ISBN-10:1118032179

10) A Penicillium sp. F33 metabolite and its synthetic derivatives inhibit acetyl-CoA:1-O-alkyl-sn-glycero-3-phosphocholine acetyltransferase (a key enzyme in platelet-activating factor biosynthesis) and carrageenan-induced paw edema in mice
Yasuhiro Yamazaki, Kengo Yasuda, Tensei Matsuyama, Takuya Ishihara, Ryoko Higa, Taira Sawairi, Masahiko Yamaguchi, Masahiro Egi, Shuji Akai, Toshio Miyase, Akira Ikari, Masao Miwa, Junko Sugatani, Biochem. Pharmacol., 86, 632–644 (2013), DOI:10.1016/j.bcp.2013.06.021
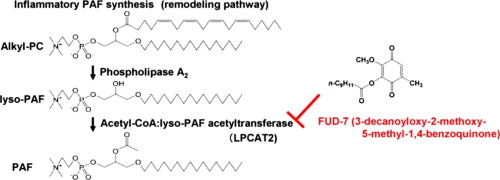
Abstract: Acetyl-CoA:1-O-alkyl-sn-glycero-3-phosphocholine (lyso-PAF) acetyltransferase is a key enzyme in the biosynthesis of 1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine (PAF) in inflammatory cells. Substances which inhibit this enzyme are of therapeutic interest. In this study, we screened for new inhibitors of lyso-PAF acetyltransferase with anti-inflammatory effects. In a metabolite from Penicillium sp. F33, we isolated an acetyltransferase inhibitor identified as dihydrofumigatin (2-methoxy-1,3,4-trihydroxy-5-methylbenzene) from high resolution mass spectrometer and NMR data. Dihydrofumigatin had strong acetyltransferase inhibitory activity, but was not stable in aqueous solution. Thus, we chemically synthesized its oxidized form fumigatin (3-hydroxy-2-methoxy-5-methyl-1,4-benzoquinone) and derivatives thereof, and evaluated their inhibitory effects. Strong inhibitory activity was observed for saturated fatty acid esters of fumigatin; the order of inhibition was 3-decanoyloxy-2-methoxy-5-methyl-1,4-benzoquinone (termed FUD-7, IC50 = 3 μM) > 2-methoxy-5-methyl-3-tetradecanoyloxy-1,4-benzoquinone (termed FUD-8, IC50 = 20 μM) > 3-hexanoyloxy-2-methoxy-5-methyl-1,4-benzoquinone (IC50 = 139 μM). Interestingly, these compounds also significantly suppressed the gene expression of lyso-PAF acetyltransferase/LPCAT2 in mouse bone marrow-derived macrophages stimulated by lipopolysaccharide (LPS). We further evaluated the effect of these substances on anti-inflammatory activity in vivo using the carrageenan-induced mouse paw edema test. FUD-7 and FUD-8 at 2.5 mg/kg showed significant, 47.9 – 51.7%, inhibition stronger than that of prednisolone at 10 mg/kg (41.9%). These results suggest that FUD-7 and FUD-8 are potent inhibitors with anti-inflammatory activity.
9) An integrated Approach to the Discovery of Potent Agelastatin A Analogues for Brain Tumors: Chemical Synthesis and Biological, Physicochemical and CNS Pharmacokinetic Analyses
Zhimin Li, Daisuke Shigeoka, Thomas R. Caulfield, Takashi Kawachi, Yushi Qiu, Takuma Kamon, Masayoshi Arai, Han W. Tun, Takehiko Yoshimitsu, Med. Chem. Commun., 4, 1093-1098 (2013), DOI:10.1039/C3MD00094J
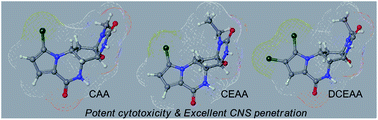
Abstract: (−)-Agelastatin A (AA), isolated from the coral sea axinellid sponge Agelas dendromorpha, has shown a high antineoplastic activity. We have synthesized eighteen AA analogues and analyzed their cytotoxicities towards three cancer cell lines. By the structure–activity relationship (SAR) study, we identified three novel analogues with higher or comparable cytotoxic activities to AA. They were subjected to chemoinformatic analysis, which revealed physicochemical properties favoring excellent central nervous system (CNS) penetration. CNS pharmacokinetic analysis in murine models validated the chemoinformatic prediction and revealed that these analogues indeed had better CNS penetration than AA. These novel potent AA analogues deserve further evaluation for therapeutic use against cancers, particularly primary and secondary brain tumors.
8) Generation of 3-borylbenzynes, their regioselective Diels–Alder reactions, and theoretical analysis
Akira Takagi, Takashi Ikawa, Yurio Kurita, Kozumo Saito, Kenji Azechi, Masahiro Egi, Yuji Itoh, Hiroaki Tokiwa, Yasuyuki Kita, Shuji Akai, Tetrahedron, 69, 4338–4352 (2013), DOI:10.1016/j.tet.2013.03.016
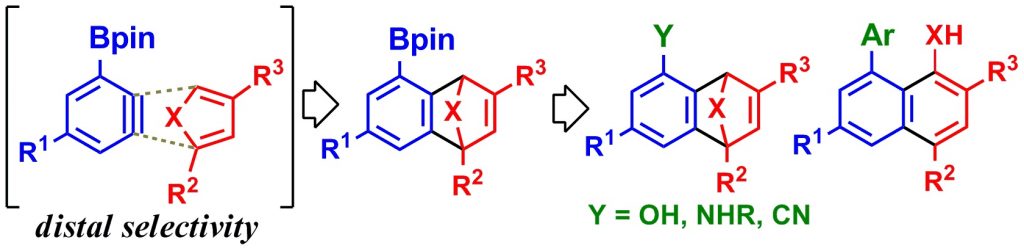
Abstract: 3-Borylbenzynes were generated in situ from 6-boryl-2-iodophenyl trifluoromethanesulfonates using i-PrMgCl·LiCl and applied to Diels–Alder reactions with substituted furans and pyrroles. The reactions allowed good functional group compatibility and produced the cycloadducts in high yields with high distal selectivities. Effective conversion of the boryl group of the products was achieved. A series of these reactions provides a new method for producing multifunctionalized benzo-fused aromatic compounds. Additionally, the regioselectivities of these Diels–Alder reactions were theoretically analyzed by DFT calculations to find that the reactions were mainly controlled by the electrostatic effect and aryne distortion caused by the boryl group.
7) Dynamic Analysis of Fluid Distribution in the Gastrointestinal Tract in Rats: Positron Emission Tomography Imaging after Oral Administration of Nonabsorbable Marker, [18F]Deoxyfluoropoly(ethylene glycol)
Tadayuki Takashima, Tomotaka Shingaki, Yumiko Katayama, Emi Hayashinaka, Yasuhiro Wada, Makoto Kataoka, Daiki Ozaki, Hisashi Doi, Masaaki Suzuki, Sho Ishida, Kentaro Hatanaka, Yuichi Sugiyama, Shuji Akai, Naoto Oku, Shinji Yamashita, Yasuyoshi Watanabe, Mol. Pharmaceutics, 10, 2261-2269 (2013), DOI:10.1021/mp300469m
Abstract: To develop potent drugs for oral use, information on their pharmacokinetic (PK) properties after oral administration is of great importance. We have recently reported the utility of positron emission tomography (PET) for the analysis of gastrointestinal (GI) absorption of radiolabeled compounds. In this study, PET image analysis was performed in rats using a novel PET probe, [18F]deoxyfluoropoly(ethylene glycol)s, with an average molecular weight of 2 kDa ([18F]FPEG), as a nonabsorbable marker to elaborate the GI physiology in more detail, such as segmental transition of the administered water, and fluid volume and distribution in the intestine. After oral administration of [18F]FPEG solution to rats, a 90 min PET scan with continuous blood sampling was performed, and then the disposition of radioactivity in each part of GI tract was investigated. From blood PK analysis, it was confirmed that the bioavailability of [18F]FPEG was quite low in rats. PET image analysis showed that the radioactivity after oral administration of [18F]FPEG solution rapidly passed through the stomach, spread into the proximal small intestine, and then transited toward the distal region of the small intestine without decreasing the radioactivity during GI transition. Radiometabolite analysis revealed that the radioactivity in intestinal mucosal tissues, blood, and urine was mainly derived from unchanged [18F]FPEG. It was also found that the volume of interest (VOI) after oral administration of the radiotracer enables an understanding of the time-dependent manner of effective fluid volume changes in the stomach and the small intestine. In addition, the rate constant of the intestinal transition of radioactivity in each intestinal segment was calculated by kinetic model analysis, which revealed that PET analysis enables us to determine the GI transit from the same individuals and that it is applicable to determine site-specific intestinal absorption. In conclusion, we demonstrated the high potency of PET imaging technique to elucidate the distribution of orally administered solution in the GI tract in vivo.
6) Formal synthesis of (−)-agelastatin A: an iron(II)-mediated cyclization strategy
Daisuke Shigeoka, Takuma Kamon, Takehiko Yoshimitsu, Beilstein Journal of Organic Chemistry, 9, 860–865 (2013), DOI:10.3762/bjoc.9.99
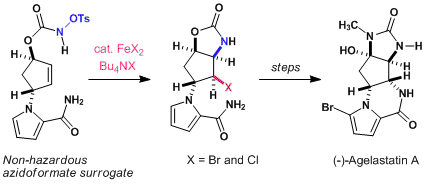
Abstract: An iron(II)-mediated aminohalogenation of a cyclopentenyl N-tosyloxycarbamate provided new access to the key intermediate for the synthesis of (−)-agelastatin A (AA, 1), a potent antiproliferative alkaloid. The present synthetic endeavour offered an insight into the mechanism underlying the iron(II)-mediated aminohalogenation of N-tosyloxycarbamate, in which the radical properties of the N–iron intermediates in the redox states were operative.
5) Discovery of aromatic components with excellent fragrance properties and biological activities: β-ionols with antimelanogenetic effects and their asymmetric syntheses
Ryoichi Komaki, Takashi Ikawa, Kozumo Saito, Kazuyo Hattori, Natsuyo Ishikawa, Hidemichi Fukawa, Masahiro Egi, Shuji Akai, Chem. Pharm. Bull., 61, 310–314 (2013) , DOI:10.1248/cpb.c12-00916
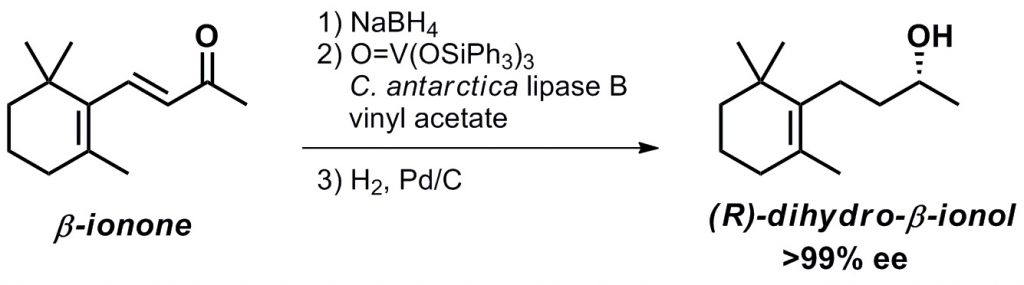
Abstract: Both enantiomers of dihydro-β-ionol and β-ionol, contained in the aromatic components of Osmanthus flower and of Hakuto peach, were obtained with high optical purity by lipase-catalyzed kinetic resolution of the racemates. It was found that all these enantiomers had different characteristic favorable scents and high antimelanogenetic effects. The absolute configuration and the enantiomer ratios of dihydro-β-ionol in the aromatic components of Osmanthus flower and of Hakuto peach were determined. The asymmetric synthesis of (R)-dihydro-β-ionol, one of the most valuable raw materials for fragrance and flavor, was performed from inexpensive β-ionone via lipase-catalyzed dynamic kinetic resolution followed by reduction.
4) Potent growth inhibitory activity of (±)-platencin towards multi-drug-resistant and extensively drug-resistant Mycobacterium tuberculosis
Gamal A. I. Moustafa, Shoji Nojima, Yoshi Yamano, Akio Aono, Masayoshi Arai, Satoshi Mitarai, Tetsuaki Tanaka, Takehiko Yoshimitsu, Med. Chem. Commun., 4, 720-723 (2013), DOI:10.1039/C3MD00016H
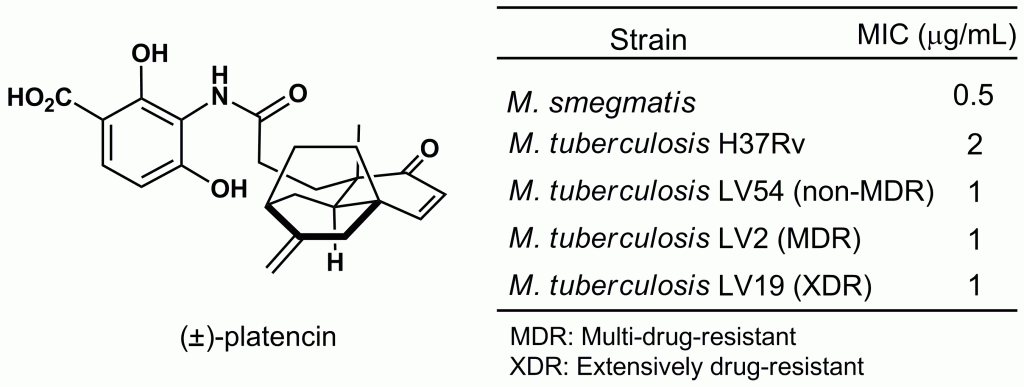
Abstract: The potent antimycobacterial activity of (+/-)-platencin is reported. Complete inhibition of Mycobacterium smegmatis growth was observed at MICs of 0.5 μg/mL and 0.3 μg/mL under aerobic and hypoxic conditions, respectively. Notably, the compound exhibited potent bacteriostatic activities towards Mycobacterium tuberculosis H37Rv (MIC=2 μg/mL), multi-drug-resistant M. tuberculosis (MIC=1 μg/mL), and extensively drug-resistant M. tuberculosis (MIC=1 μg/mL). An overexpression study of the transformants of M. smegmatis revealed that platencin selectively targeted Mt-KasB and modestly inhibited Mt-KasA and Mt-FabH.
3) Regiocomplementary Cycloaddition Reactions of Boryl- and Silylbenzynes with 1,3-Dipoles: Selective Synthesis of Benzo-Fused Azole Derivatives
Takashi Ikawa, Akira Takagi, Masahiko Goto, Yuya Aoyama, Yoshinobu Ishikawa, Yuji Itoh, Satoshi Fujii, Hiroaki Tokiwa, Shuji Akai, J. Org. Chem., 78, 2965–2983 (2013), DOI:10.1021/jo302802b
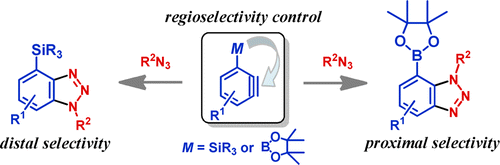
Abstract: Benzo-fused nitrogen-containing heterocycles are abundant in biologically active compounds. One of the most important methods for preparing such heterocycles is the (3 + 2) cycloaddition reaction of benzynes with 1,3-dipolar compounds. However, the reactions of unsymmetrically substituted benzynes generally show low selectivity and hence yield mixtures of two regioisomers. In this paper, we describe the synthesis of both regioisomers of multisubstituted benzo-fused azole derivatives such as benzotriazoles, 1H-indazoles, and benzo[d]isoxazoles through the regiocomplementary (3 + 2) cycloaddition reactions of 3-boryl- and 3-silylbenzynes with 1,3-dipoles. The improved generation of 3-borylbenzynes from new precursors was one of the most important results of this work, which produced the successful (3 + 2) cycloaddition reactions with exclusive and proximal selectivities. On the other hand, similar reactions of 3-silylbenzynes selectively afforded distal cycloadducts. Analysis of the reaction pathways of these amazing regioselectivities by density functional theory calculations revealed that the (3 + 2) cycloadditions of borylbenzynes are controlled by the electrostatic effect of the boryl group, while those of silylbenzynes are controlled mainly by the steric effect of the bulky silyl groups that produced electrostatically unfavorable adducts via anomalous transition states.
2) A Mesoporous-Silica-Immobilized Oxovanadium Cocatalyst for the Lipase-Catalyzed Dynamic Kinetic Resolution of Racemic Alcohols
Masahiro Egi, Koji Sugiyama, Moriaki Saneto, Ryosuke Hanada, Katsuya Kato, Shuji Akai, Angew. Chem. Int. Ed., 52, 3654–3658 (2013), DOI:10.1002/anie.201208988
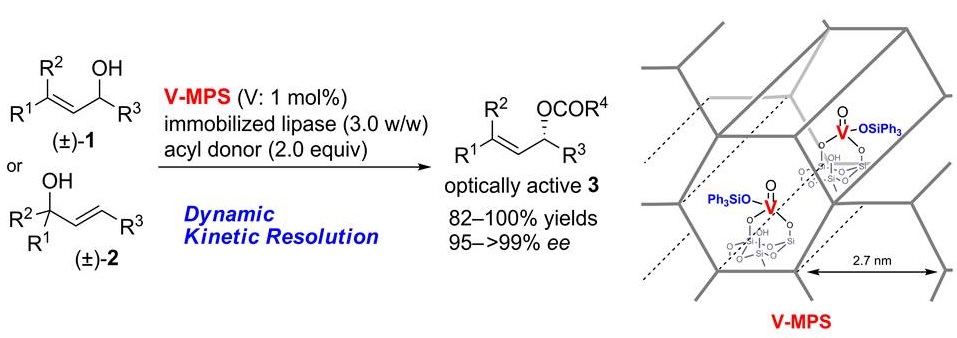
Abstract: A new oxovanadium catalyst (V-MPS; see scheme) immobilized in the pores of mesoporous silica has been developed. The combined use of V-MPS and lipases achieved the dynamic kinetic resolution of a wide range of racemic alcohols (1 or 2) to produce optically active esters 3 in high chemical and optical yields. The paired catalysts retained high catalytic activity when reused up to six times.
1) Dichlorination of olefins with NCS/Ph3P
Yasumasa Kamada, Yuta Kitamura, Tetsuaki Tanaka, Takehiko Yoshimitsu, Org. Biomol. Chem., 11, 1598-1601 (2013), DOI:10.1039/C3OB27345H
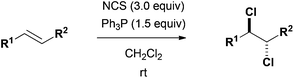
Abstract: A 2:1 mixture of NCS and Ph3P successfully promoted the anti-dichlorination of olefins to provide corresponding dichlorides, serving as a molecular chlorine surrogate generated in situ.