4) Correlation of indoleamine-2,3-dioxigenase 1 inhibitory activity of 4,6-disubstituted indazole derivatives and their heme binding affinity
Hirofumi Tsujino, Tadayuki Uno, Taku Yamashita, Masafumi Katsuda, Kazuki Takada, Takeshi Saiki, Shotaro Maeda, Akira Takagi, Shigeaki Masuda, Yasuhiko Kawano, Kanji Meguro, Shuji Akai
Bioorg Med Chem Lett., 2019, 29, 126607.
https://doi.org/10.1016/j.bmcl.2019.08.011
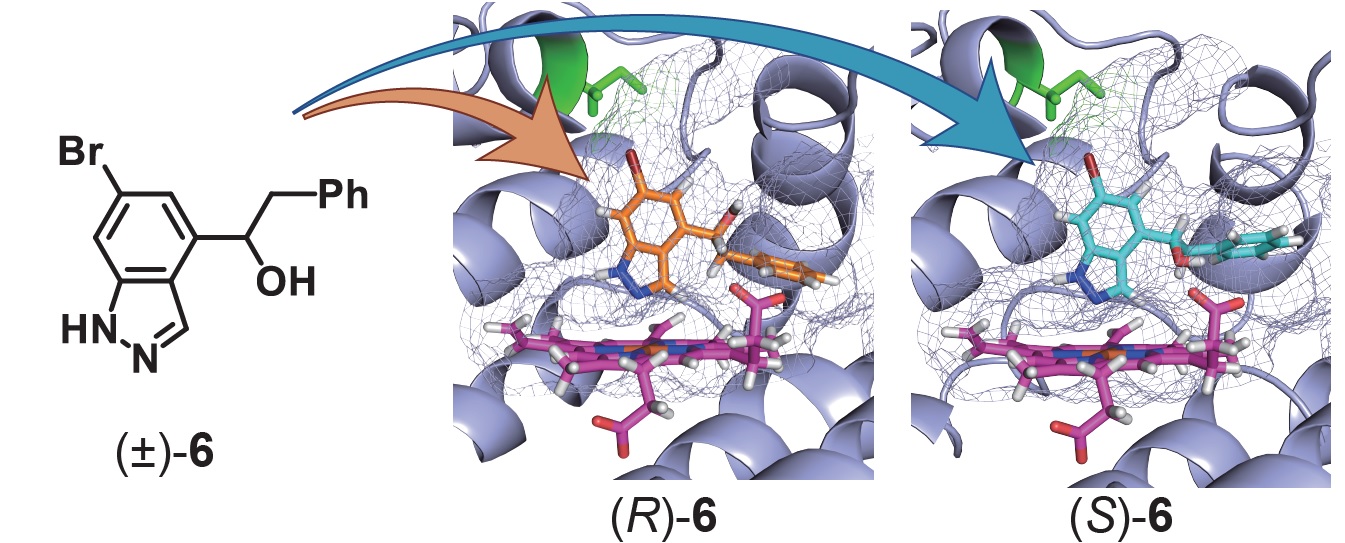
Abstract: Indoleamine 2,3-dioxygenase 1 (IDO1) is a heme-containing enzyme that acts on the first and rate-limiting step of the tryptophan/kynurenine pathway. Since the pathway is one of the means of cancer immune evasion, IDO1 inhibitors have drawn interest as potential therapeutics for cancers. We found a 4,6-disubstituted indazole 1 as a hit compound that showed both IDO1 inhibitory activity and binding affinity for IDO1 heme. Structural modification of 1 yielded compound 6, whose relatively large substituent at the 4-position and proper size substituent at the 6-position were found to be important for the enhancement of IDO1 inhibitory activity and heme affinity. A series of compounds synthesized in this work were evaluated by in silico docking simulations and by in vitro experiments using a C129Y mutant of the pocket-A of IDO1. Our results revealed that proper substituents at the 6- and 4-positions of the compounds interact with pockets A and B, respectively, and that, in particular, a good fit in pocket-A is important for the compounds’ biological activities. Absorption spectral analysis of these compounds showed that they strongly bound to the ferrous heme rather than its ferric heme. Furthermore, we observed that the heme affinities of these compounds strongly correlate with their IDO1 inhibitory activities.
3) Ammonium Salt-Accelerated Hydrazinolysis of Unactivated Amides: Mechanistic Investigation and Application to a Microwave Flow Process
Megumi Noshita, Yuhei Shimizu, Hiroyuki Morimoto, Shuji Akai, Yoshitaka Hamashima, Noriyuki Ohneda, Hiromichi Odajima, and Takashi Ohshima
Org. Process Res. Dev., 2019, 23, 588–594.
DOI: 10.1021/acs.oprd.8b00424
Abstract: A study of ammonium salt-accelerated hydrazinolysis of unactivated amides is described. We first studied the reaction mechanism by kinetic experiments and DFT calculations and found that cooperation of the hydrazinium salt and hydrazine is important for promoting the cleavage of unactivated amides. Next, we applied the reaction to a microwave flow process, and the amine products were isolated on a multigram scale.
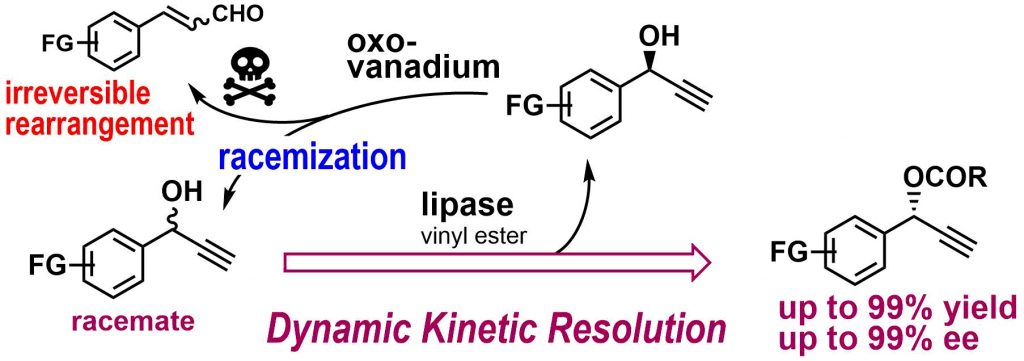
2) Lipase/Oxovanadium Co-Catalyzed Dynamic Kinetic Resolution of 2 Propargyl Alcohols: Competition between Racemization and Rearrangement
Shinji Kawanishi, Shinya Oki, Dhiman Kundu, Shuji Akai
Org. Lett., 2019, 21, 2978−2982.
DOI: 10.1021/acs.orglett.9b00334
Cited as a Cover Picture
Abstract: Quantitative conversion of racemic propargyl alcohols into optically active propargyl esters with up to 99% ee has been achieved by lipase/oxovanadium co-catalyzed dynamic kinetic resolution, which combines the lipase-catalyzed enantioselective esterification of the racemic substrates and the in-situ racemization of the remaining enantiomers. The success is owing to our discovery of a magic solvent, (trifluoromethyl)benzene, that accelerated the racemization while sufficiently suppressing the common oxovanadium-catalyzed rearrangement of propargyl alcohols to irreversibly produce enals.
1) Base-promoted lipase-catalyzed kinetic resolution of atropisomeric 1,1’-biaryl-2,2’-diols
Gamal A. I. Moustafa, Kengo Kasama, Koichi Higashio, Shuji Akai
RSC Adv., 2019, 9, 1165–1175.
DOI: 10.1039/c8ra09070j
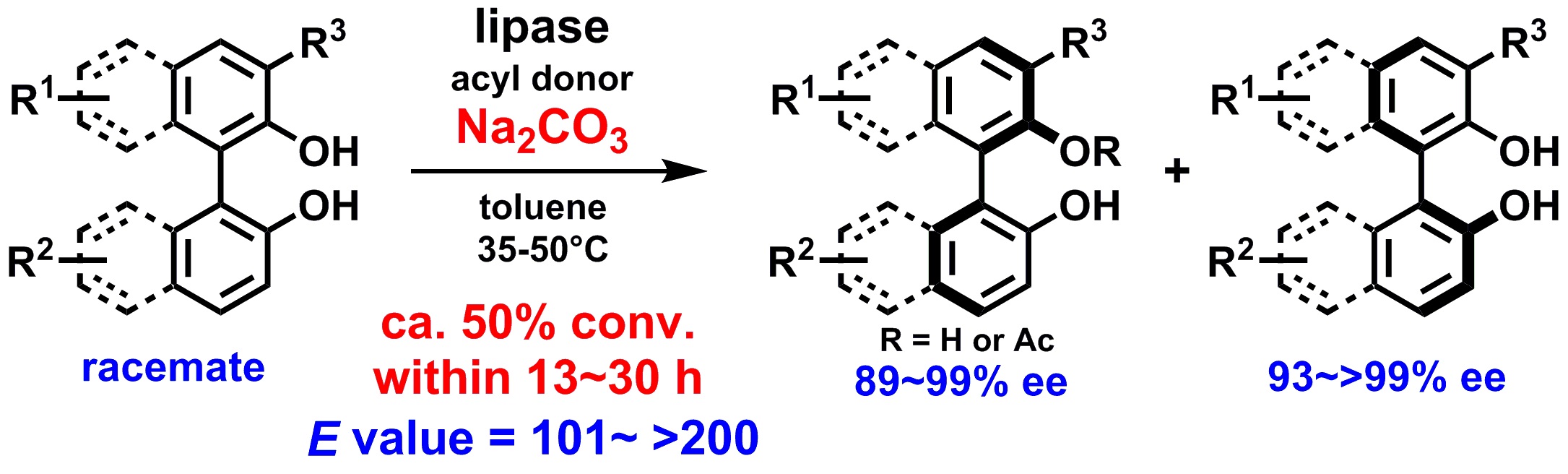
Abstract: Herein we report a dramatic acceleration of the lipase-catalyzed kinetic resolution of atropisomeric 1,1’-biaryl-2,2’-diols by the addition of sodium carbonate. This result likely originates from the increased nucleophilicity of the phenolic hydroxyl group toward the acyl-enzyme intermediate. Under these conditions, various substituted C2-symmetric and non-C2-symmetric binaphthols and biphenols were efficiently resolved with ~50% conversion in only 13–30 h with excellent enantioselectivity.