12) Versatile Biaryls and Fused Aromatics through Oxidative Coupling of Hydroquinones with (Hetero)Arenes
Takaaki Aijima, Rina Ueda, Takanori Nakane, Fumiaki Makino, Yusuke Ohnishi, Keiichiro Nakajima, Shinichiro Kamino, Genji Kurisu, Keiichi Namba, Hiroki Nakata, Kaiki Mogi, Hironao Sajiki, Shuji Akai and Yoshinari Sawama*
ChemRxiv, 2023
DOI: 10.26434/chemrxiv-2023-7pf7h
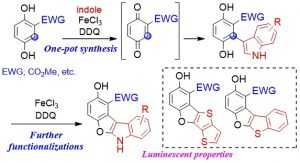
Abstract: Hydroquinones bearing an electron-withdrawing group at the C2-position effectively underwent oxidative coupling with indoles or arenes in the presence of 2,3-dichloro-5,6-dicyano p-benzoquinone (DDQ) and FeCl3 to give the corresponding biaryls. Indole-based products were further converted into tetracyclic aromatics using DDQ and FeCl3. Thiophene derivatives were also applicable to give the tetracyclic aromatics, possessing luminescent properties.

11) Construction of Functionalized ortho-Naphthoquinone Methides via Site-Selective Ring Opening of 1-Siloxy-1,4-epoxy-1,4-dihydronaphthalenes
Takaaki Aijima, Shinsuke Komagawa, Shuji Akai, and Yoshinari Sawama*
Adv. Synth. Catal. 2023, 365, 3981– 3986
DOI:10.1002/adsc.202300968
大阪大学リポジトリOUKA(オープンアクセス)https://hdl.handle.net/11094/93212
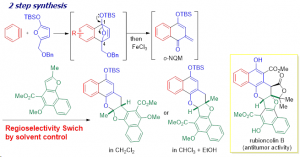
Abstract: 1-Siloxy-4-(benzyloxy)methyl-1,4-epoxy-1,4-dihydronaphthalenes, generated from benzynes and furans, underwent automatic site-selective ring opening because of the synergetic effect of the steric strain of the 1,4-epoxy moiety and the electron-donating ability of the siloxy group on the acetal structure to afford the precursors of ortho-naphthoquinone methides (o-NQMs). Subsequent Lewis acid-facilitated o-NQM formation and annulation with olefins afforded multi-fused heterocycles. Notably, the consecutive hexacyclic skeleton of rubioncolin B was constructed via solvent-dependent regioselective annulation of naphthofuran derivatives.

10) Impact of Multiple H/D Replacement on the Physicochemical Properties of Flurbiprofen
Hiromasa Uchiyama, Kazuho Ban, Shiho Nozaki, Yui Ikeda, Takayoshi Ishimoto, Hiroyoshi Fujioka, Mako Kamiya, Ryugo Amari, Hirofumi Tsujino, Masayoshi Arai, Sachi Yamazoe, Keiko Maekawa, Takuma Kato, Mitsunobu Doi, Kazunori Kadota, Yuichi Tozuka, Naohito Tomita, Hironao Sajiki, Shuji Akai, Yoshinari Sawama
RSC Med. Chem. 2023, 14, 2583–2592
DOI: 10.1039/d3md00357d
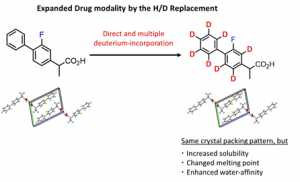

Abstract: Although deuterium incorporation into pharmaceutical drugs is an attractive way to expand drug modalities, their physicochemical properties have not been sufficiently examined. This study focuses on examining the changes in physicochemical properties between flurbiprofen (FP) and flurbiprofen-d8 (FP-d8), which was successfully prepared by direct and multiple H/D exchange reactions at the eight aromatic C-H bonds of FP. Although the effect of deuterium incorporation was not observed between the crystal structures of FP and FP-d8, the melting point and heat of fusion of FP-d8 were lower than those of FP. Additionally, the solubility of FP-d8 increased by 2-fold compared to that of FP. Calculation of the interaction energy between FP/FP-d8 and water molecules using the multi-component density functional theory method resulted in increased solubility of FP-d8. These novel and valuable findings regarding the changes in physicochemical properties triggered by deuterium incorporation can contribute to the further development of deuterated drugs.

9) Oxidative Functionalization of Catechol Derivatives Substituted with Electron-withdrawing Groups
Yoshinari Sawama Hyoga Shimizu, Takaaki Aijima, Taro Udagawa, Shoko Kuwata, Tsuyoshi Yamada, Hironao Sajiki, and Shuji Akai
Chem. Pharm. Bull. .2023, 71, 782-786
DOI: 10.1248/cpb.c23-00493
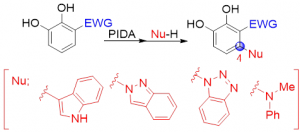
Abstract: Catechols possessing electron-withdrawing groups at the C3 position effectively underwent oxidative functionalization at the C4 position in the presence of phenyliodine(III) diacetate (PIDA) and heteroarene nucleophiles (e.g., indole, indazole, and benzotriazole) to produce the corresponding biaryl products. The PIDA-mediated oxidation of catechol derivatives afforded the ortho-benzoquinone intermediate, which subsequently underwent regioselective nucleophilic addition to the a,b-unsaturated carbonyl moiety of ortho-benzoquinone using indole, indazole, and benzotriazole to give 4-substituted catechol derivatives in a one-pot manner. Notably, the nucleophilic substitution positions of indazole and benzotriazole were perfectly controlled. Additionally, the reaction using N-methylaniline as the nucleophile afforded a tertiary amine product.

8) Sulfonium Salt Reagents for the Introduction of Deuterated Alkyl Groups in Drug Discovery
Kazuho Ban, Keisuke Imai, Shuki Oyama, Jin Tokunaga, Yui Ikeda, Hiromasa Uchiyama, Kazunori Kadota, Yuichi Tozuka, Shuji Akai, and Yoshinari Sawama
Angew. Chem. Int. Ed. 2023, e202311058, cited as frontspiece in ACIE, SYNFACTS, and Month’s Highlight in OPR&D.
DOI: 10.1002/anie.202311058
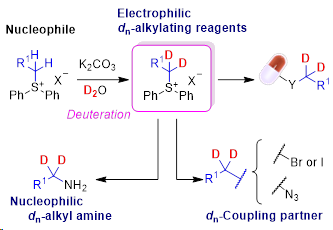

Abstract: The pharmacokinetics of pharmaceutical drugs can be improved by replacing C- H bonds with the more stable C-D bonds at the α-position to heteroatoms, which is a typical metabolic site for cytochrome P450 enzymes. However, the application of deuterated syn-thons is limited. Herein, we established a novel concept for preparing deuterated reagents for the successful synthesis of complex drug skeletons with deuterium atoms at the α-position to heteroatoms. (dn-Alkyl)-diphenylsulfonium salts prepared from the corresponding nondeuterated forms using inexpensive and abundant D2O as the deuterium source with a base, were used as electrophilic alkylating reagents. Additionally, these deuterated sulfonium salts were efficiently trans-formed into dn-alkyl halides and a dn-alkyl azide as coupling reagents and a dn-alkyl amine as a nucleophile. Furthermore, liver microsomal metabolism studies revealed deuterium kinetic isotope effects (KIE) in 7-(d2-ethoxy)flavone. The present concept for the synthesis of deuterated reagents and the first demonstration of a KIE in a d2-ethoxy group will contribute to drug discovery research based on deuterium chemistry.

7) Multiple deuteration of triphenylphosphine and live-cell Raman imaging
of deuterium-incorporated Mito-Q
Shogo Moriyama, Miyu Mae, Daiki Shibata, Hiroyuki Yamakoshi, Shinji Kajimoto, Takakazu Nakabayashi, Takayoshi Ishimoto, Kaiki Mogi, Hironao Sajiki, Shuji Akai, Yoshinari Sawama
Chem. Commun. 2023, 59, 12100 – 12103, Cited as a cover art.
DOI: 10.1039/d3cc04410f
ラマン解析のデータがSSBD:databaseで公開されました。
https://ssbd.riken.jp/database/project/369/
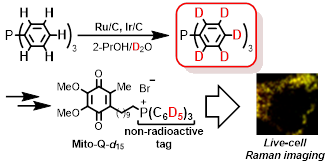

Abstract: All aromatic C–H bonds of triphenylphosphine (PPh3) were effi-ciently replaced by C–D bonds using Ru/C and Ir/C co-catalysts in 2-PrOH and D2O, an inexpensive deuterium source. Furthermore, non-radioactive and safe deuterium-incorporated Mito-Q (drug candidate) was prepared from deuterated PPh3 and used for the live-cell Raman imaging to evaluate the mitochondrial uptake.

6) C-O結合の均等開裂を伴うアルコールのone-pot官能基化
One─pot Functionalization of Alcohols via Homolytic Cleavage of their C─O Bonds
森山将吾
有機合成化学協会誌, 2023 年 81 巻 9 号 p. 892-894
DOI:10.5059/yukigoseikyokaishi.81.892
Abstract: The Barton-McCombie type reaction usually requires prefunctionalization of the hydroxy group of alcohols to induce deoxygenative generation of alkyl radicals. In recent years, synthetic reactions, wherein reactive intermediates generated in situ induce homolytic C-O bond cleavage of alcohols, have been reported. The present short review will discuss recent progress in radical alkylation using alcohols as alkyl radical sources.
5) Lipase/H2SO4-Cocatalyzed Dynamic Kinetic Resolution of Alcohols in Pickering Emulsion
Jihoon Moon, Takusho Kin, Karin Mizuno, Shuji Akai, Kyohei Kanomata
ChemCatChem, 2023, 15, e202300878.
DOI:10.1002/cctc.202300878
大阪大学リポジトリOUKA(オープンアクセス)https://hdl.handle.net/11094/94659
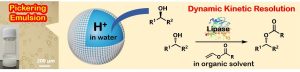
Abstract: This study reports the first chemoenzymatic dynamic kinetic resolution (DKR) of racemic sec-alcohols by simultaneously using immobilized lipase and aqueous sulfuric acid as catalysts for kinetic resolution and racemization, respectively. The nanoparticle-stabilized phase separation in a Pickering emulsion enabled the use of these inherently incompatible catalysts in a single vessel. The racemization reaction in the aqueous sulfuric acid solution significantly suppressed the dehydrative side reactions that form alkenes and dimeric ethers, which are often observed in racemization using Brønsted/Lewis acids or oxovanadium compounds in organic solvents. The efficacy of the Pickering emulsion was confirmed by the significantly lower catalytic performance in DKR without emulsification. This method achieved high yields and high optical purities in the production of a wide range of sec-alcohols.

4) Heterogeneously Catalyzed Aromatic Reduction
Yoshinari Sawama, Kazuho Ban, Hironao Sajiki
Industrial Arene Chemistry: Markets, Technologies, Sustainable Processes and Cases Studies of Aromatic Commodities (Chapter 30), Jacques Mortier, Wiley‐VCH GmbH, 2, 883-918 (2023)
DOI:10.1002/9783527827992.ch30

3) Unprecedented Regioselective Deuterium-Incorporation of Alkyltrimethylammonium Chlorides and Raman Analysis
Yoshinari Sawama, Takumi Matsuda, Shogo Moriyama, Kazuho Ban, Hiroyoshi Fujioka, Mako Kamiya, Jingwen Shou, Yasuyuki Ozeki, Shuji Akai, Hironao Sajiki
Asian J. Org., Chem. 2023, e202200710.
DOI:10.1002/ajoc.202200710
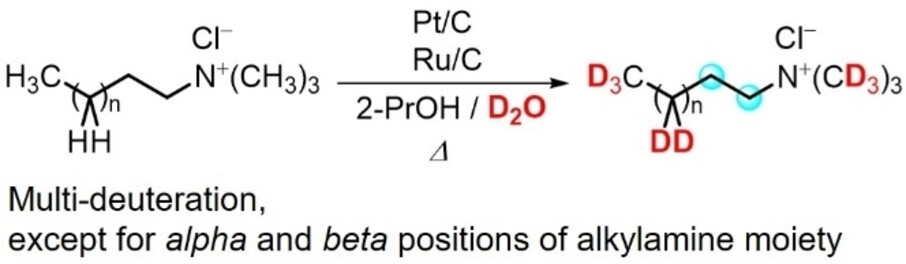
Abstract: Alkyltrimethylammonium chlorides (ATACs) are widely utilized as cationic surfactants in various scientific fields. Furthermore, deuterium-incorporated compounds can be powerful tools for elucidating the complicated morphologies of molecules with high molecular weights. Dodecyltrimethylammonium chloride (DTAC), as a representative ATAC, underwent the regioselective multi-deuteration of alkylamine moiety, except for α and β-positions. Deuteration was performed under Pt/C and Ru/C-catalyzed conditions in 2-PrOH and D2O. The stimulated Raman scattering (SRS) revealed the specific signals of multi-deuterated DTAC in the silent region.

2) 機械学習で予測するC(sp3)–H官能基化反応の位置選択性
鹿又喬平
ファルマシア, 2023, 59, 5, 432
DOI:10.14894/faruawpsj.59.5_432
新規な基質の反応位置・立体選択性などを予測するツールとして,機械学習を利用する試みが活発化している.C–H結合の官能基化反応では,配向基を用いず,触媒のみによって制御される反応の位置選択性を経験的に予測することは難しい.最近Boniらは,二核ロジウム錯体を触媒とするC(sp3)–H変換反応について,機械学習による位置選択性の予測モデルを報告したので紹介する.
なお,本稿は下記の文献に基づいて,その研究成果を紹介するものである.
Boni Y. T. et al., J. Am. Chem. Soc., 144, 15549–15561 (2022).

1) Chemoenzymatic Dynamic Kinetic Resolution of Alcohols
Kyohei Kanomata, Shuji Akai
Science of Synthesis, 2022/4: Dynamic Kinetic Resolution (DKR) and Dynamic Kinetic Asymmetric Transformations (DYKAT): Bäckvall, J.-E., Ed.; Thieme: Stuttgart (2023), Vol. 1, p 181–217.
DOI: 10.1055/sos-SD-237-00069
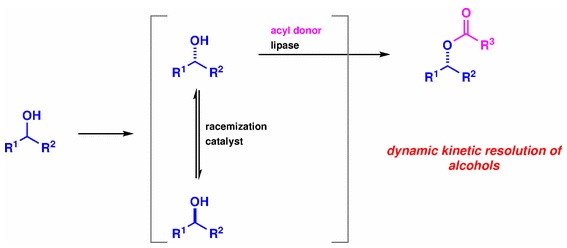
Abstract: Chemoenzymatic dynamic kinetic resolution is one of the simplest and most reliable methods to obtain optically pure alcohol derivatives from racemates. For this purpose, hydrolases, especially lipases, have been widely used in the enantioselective esterification processes, and a variety of racemization catalysts with high catalytic efficiency and compatibility with lipases have been developed. This review introduces chemoenzymatic DKR of alcohols based on the category of racemization catalysts. DKR of axially chiral hydroxybiaryls and the use of engineered lipases to obtain opposite enantiomers, as well as the synthetic applications of the DKR products, are also discussed.