14) Improved In Vivo Delivery of Small RNA Based on the Calcium Phosphate Method
Xin Wu, Yuhki Yokoyama, Hidekazu Takahashi, Shihori Kouda, Hiroyuki Yamamoto, Jiaqi Wang, Yoshihiro Morimoto, Kazumasa Minami, Tsuyoshi Hata, Awad Shamma, Akira Inoue, Masahisa Ohtsuka, Satoshi Shibata, Shogo Kobayashi, Shuji Akai, and Hirofumi Yamamoto
J. Pers. Med. 2021, 11, 1160.
https://doi.org/10.3390/jpm11111160
Abstract: In the past few years, we have demonstrated the efficacy of a nanoparticle system, super carbonate apatite (sCA), for the in vivo delivery of siRNA/miRNA. Intravenous injection of sCA loaded with small RNAs results in safe, high tumor delivery in mouse models. To further improve the efficiency of tumor delivery and avoid liver toxicity, we successfully developed an inorganic nanoparticle device (iNaD) via high-frequency ultrasonic pulverization combined with PEG blending during the production of sCA. Compared to sCA loaded with 24 µg of miRNA, systemic administration of iNaD loaded with 0.75 µg of miRNA demonstrated similar delivery efficiency to mouse tumors with little accumulation in the liver. In the mouse therapeutic model, iNaD loaded with 3 µg of the tumor suppressor small RNA MIRTX resulted in an improved anti-tumor effect compared to sCA loaded with 24 µg. Our findings on the bio-distribution and therapeutic effect of iNaD provide new perspectives for future nanomedicine engineering.
13) 医薬品合成を指向した酵素触媒連続フロー合成
Continuous flow biocatalysis for the synthesis of pharmaceuticals
赤井周司 Shuji Akai
化学工学, 2021, 85, 619-622; Chemical Engineering Japan, 2021, 85, 619-622
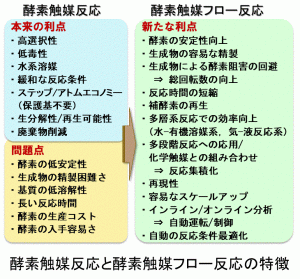
グリーンケミストリーの理念に則った持続可能な生産プロセス開発の必要性と相まって,常温常圧の温和な条件下,高効率的で選択性の高い酵素を触媒とする変換プロセスは,産業界や学術界において関心が高まっている。さらに,近年のバイオテクノロジー,タンパク質工学,酵素の指向性進化技術,計算科学などのめざましい進歩は,酵素触媒反応の実践性を大きく向上させた。今日では,目的の反応に最適のテーラーメイド酵素を,わずか数か月で調製することも可能になった。さらに,連続フロー合成技術との融合によって,古典的なバッチ法での酵素触媒反応の諸問題が一挙に解決できる可能が高まっている。本稿では,医薬原体,天然有機化合物,ならびにこれらの合成中間体の酵素触媒連続フロー合成法の最近の進歩を紹介したい。
12) Chemo- and regioselective cross-dehydrogenative coupling reaction of 3-hydroxycarbazoles with arenols catalyzed by a mesoporous silica-supported oxovanadium
Kengo Kasama, Kyohei Kanomata, Yuya Hinami, Karin Mizuno, Yuta Uetake, Toru Amaya, Makoto Sako, Shinobu Takizawa, Hiroaki Sasai and Shuji Akai*
RSC Adv., 2021, 11, 35342–35350.
DOI: 10.1039/d1ra07723f
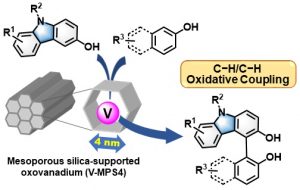
Abstract: Cross-dehydrogenative coupling between 3-hydroxycarbazoles and 2-naphthols has been achieved by using a mesoporous silica-supported oxovanadium catalyst.
11) フローマイクロ合成の最新動向 Recent Trends in Flow Microreactor Synthesis
赤井 周司 (Shuji Akai) 他著
深瀬 浩一, 永木 愛一郎監修, シーエムシー出版, ISBN 978-4-7813-1615-4, 発行 2021年8月31日

10) Could London Dispersion Force Control Regioselective (2 + 2) Cyclodimerizations of Benzynes? YES: Application to the Synthesis of Helical Biphenylenes
Takashi Ikawa,* Yuta Yamamoto, Akito Heguri, Yutaka Fukumoto, Tomonari Murakami, Akira Takagi, Yuto Masuda, Kenzo Yahata, Hiroshi Aoyama, Yasuteru Shigeta, Hiroaki Tokiwa,* and Shuji Akai*
J. Am. Chem. Soc. 2021, 143, 10853−10859. Cited as a cover picture.
https://doi.org/10.1021/jacs.1c05434
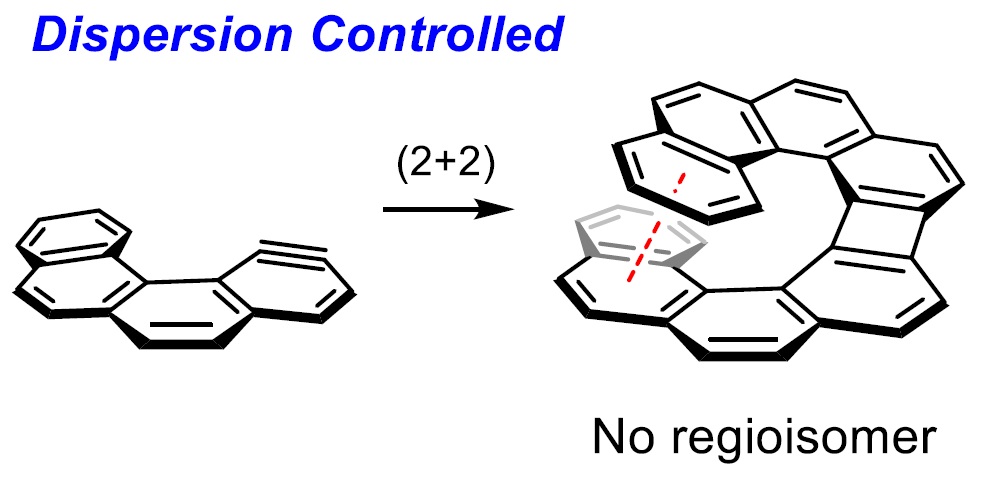

Abstract: In recent years, London dispersion interactions, which are the attractive component of the van der Waals potential, have been found to play an important role in controlling the regio- and/or stereoselectivity of various reactions. Particularly, the dispersion interactions between substrates and catalysts (or ligands) are dominant in various selective catalyzes. In contrast, repulsive steric interactions, rather than the attractive dispersion interactions, between bulky substituents are predominant in most of the noncatalytic reactions. Herein, we demonstrate the first example of London dispersion-controlled noncatalytic (2 + 2) cyclodimerization of substituted benzynes to selectively afford proximal biphenylenes in high yields and regioselectivities, depending on the extent of dispersion interactions in the substituents. This method can be applied for the synthesis of novel helical biphenylenes, which would be fascinating for chemists as these compounds are potential skeletons for ligands, catalysts, and medicines.
9) Direct nucleophilic substitution of alcohols using an immobilized oxovanadium catalyst
Tomoya Nishio, Shin Yoshioka, Kai Hasegawa, Kenzo Yahata, Kyohei Kanomata, and Shuji Akai*
Eur. J. Org. Chem., 2021, 4417–4422. Cited as a cover picture.
DOI:10.1002/ejoc.202100569
大阪大学リポジトリOUKA(オープンアクセス)https://hdl.handle.net/11094/92243
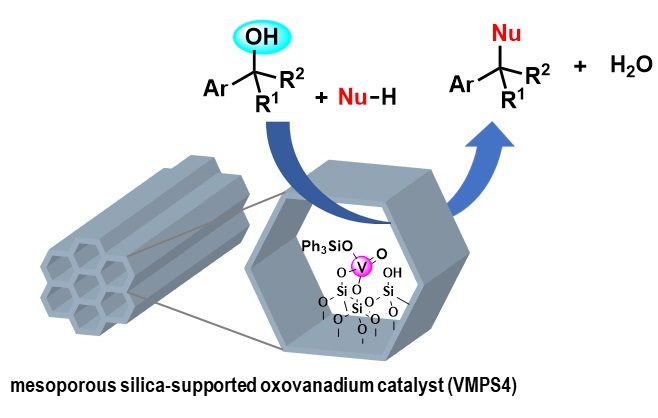

Abstract: Direct nucleophilic substitution of alcohols with thiols or carbon nucleophiles was achieved using a mesoporous silica-supported oxovanadium catalyst (VMPS4). Benzyl and allyl alcohols were compatible in this reaction under mild conditions, affording the products in high yields. The VMPS4 catalyst showed excellent chemoselectivity toward alcohols in the presence of acid-labile functional groups, which is in contrast to that observed for the commonly used Lewis acid catalysts, which exhibit poor selectivity. The VMPS4 catalyst could be recycled by simple centrifugation, and the catalytic activity was maintained over seven cycles.
8) Gold-Catalyzed Tandem Oxidative Coupling Reaction between β-Ketoallenes and Electron-Rich Arenes to 2-Furylmethylarenes
Naoki Yasukawa, Yutaro Yamada, Chikara Furugen, Yuya Miki, Hironao Sajiki, Yoshinari Sawama
Organic Letters, 2021, 23, 15, 5891-5895
DOI:10.1021/acs.orglett.1c02007
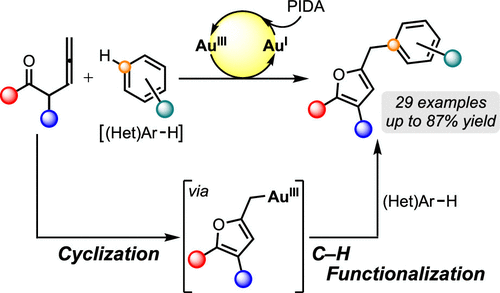
Abstract: A tandem oxidative coupling reaction of β-ketoallenes and arenes was developed, which leads to the formation of 2-furylmethylarenes using AuCl3 and phenyliodine diacetate. The AuIII salt catalyzed the cyclization of β-ketoallenes to form a 2-furylmethyl gold intermediate, and the subsequent C–H functionalization of arenes proceeded smoothly. During the oxidative coupling, nucleophilic additions occurred at the center and terminal carbon atoms of the allene moiety to form C–O and C–C bonds.
7) Synthesis of 1-Pyrroline by Denitrogenative Ring Expansion of Cyclobutyl Azides under Thermal Conditions
Yuya Miki, Naohito Tomita, Kazuho Ban, Hironao Sajiki, Yoshinari Sawama
Advanced Synthesis & Catalysis, 2021, 363, 14, 3481-3484
DOI:10.1002/adsc.202100329
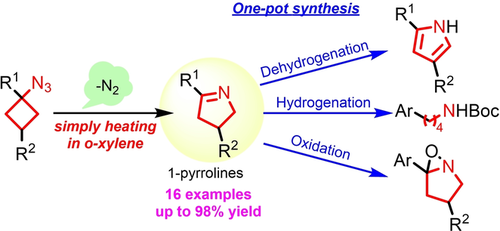
Abstract: We herein report an efficient and systematic synthesis of 1-pyrrolines from cyclobutyl azides under thermal and neutral conditions. The reaction proceeded without any additional reagents, and nitrogen was generated as the sole by-product. Furthermore, the generated 1-pyrrolines could be continuously transformed into pyrroles, N-Boc-amines, and oxaziridines in an one-pot manner.
6) Synthetic Studies on the Viridin Skeleton through Regio- and Stereoselective Functionalization of the AE-Ring Moiety
Shuhei Hori, Sho Ishida, Go Itoh, Koji Sugiyama, Chiharu Yuki, Masahiro Egi, Kenzo Yahata, Takashi Ikawa, Shuji Akai
Synlett, 2021, 32, 1187-1191
DOI: 10.1055/a-1527-3781
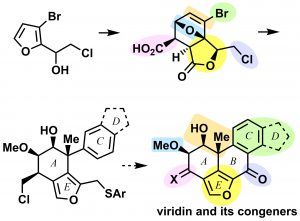
Abstract: 4,5,6,7-Tetrahydroisobenzofurans, corresponding to the AC(D)E ring structure of viridin and equipped with required substituents on the A-ring, were synthesized via the Diels-Alder adduct of a furan derivative and maleic anhydride with high regio- and stereoselectivities. The key steps of this work include the regioselective opening of the tetrahydrofuran, stereoselective epoxidation, and AlMe3-mediated regioselective epoxide opening followed by stereoselective C-methylation.
5) Review de Debut “Redox-neutral Mitsunobu Reaction”
笠間建吾 Kengo Kasama
有機合成化学協会誌, 2021, 79, 344-345.
Synth. Org. Chem. Japan, 2021, 79, 344-345.
DOI: https://doi.org/10.5059/yukigoseikyokaishi.79.344
Abstract: More than 50 years after the original Mitsunobu reaction reported in 1967, its catalytic and redox-neutral reactions have been developed. While the conventional method has problems such as the laborious separation of by-products and the explosive nature of azo reagents, the redox-neutral method solves these problems and its catalytic method enabled water as a sole by-product. This article describes the recent developments in the redox-neutral Mitsunobu reactions.
4) 1-Alkoxyvinyl Ester as an Excellent Acyl Donor: Efficient Macrolactone Synthesis
Yasuyuki Kita*, Shuji Akai, Hiromichi Fujioka, and Tohru Kamitanaka
J. Org. Chem. 2021, 86, 5, 3683–3696.Cited as a cover picture.
DOI: https://doi.org/10.1021/acs.joc.0c02677
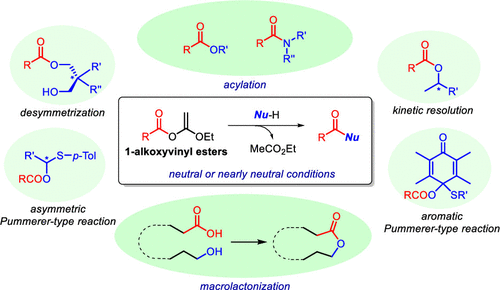

Abstract: Ketene acetal derivatives, such as 1-alkoxyvinyl esters and O-silyl ketene acetals, belong to the category of O-substituted enols of esters, which easily react with various types of nucleophiles, Nu–H, under neutral conditions to give the corresponding acylated and silylated products in excellent yields only by evaporation of the generated volatile esters. Silyl ketene acetals can be easily synthesized by various simple procedures, whereas 1-alkoxyvinyl esters require an equimolar or catalytic amount of a mercury salt to synthesize them. This drawback prevented the advancement of the chemistry of 1-alkoxyvinyl esters. In 1993, we developed a useful synthetic method of 1-alkoxyvinyl esters using a small amount (0.5–1 mol %) of a ruthenium catalyst. Encouraged by this discovery, we subsequently developed various reactions and applied them to the synthesis of natural products. It is noteworthy that the stereoselective total synthesis of fredericamycin A was achieved by the combined use of these reactions. Macrocyclization was variously utilized for the synthesis of natural macrolides by two types of approaches: direct macrolactonization of α,ω-hydroxy acids or intermolecular esterification between an acid and alcohol followed by a ring-closure step. Additionally, several new reactions using 1-alkoxyvinyl esters or their analogs as key intermediates on the basis of our methods were recently reported. In this paper, we introduce our efforts from the synthesis of 1-alkoxyvinyl esters to the application such as natural product syntheses and recent advancements.
3) 巻頭言 「もっと日本語を大切に」Prefatory note: “Valuing the Japanese Language More”
赤井周司 Shuji Akai
有機合成化学協会誌, 2021, 79, 99.
Synth. Org. Chem. Japan, 2021, 79, 99.
DOI: https://doi.org/10.5059/yukigoseikyokaishi.79.99
2) Four-Step One-Pot Catalytic Asymmetric Synthesis of Polysubstituted Tricyclic Compounds: Lipase-Catalyzed Dynamic Kinetic Resolution Followed by an Intramolecular Diels–Alder Reaction
Izuru Tsuchimochi, Shuhei Hori, Yasuo Takeuchi, Masahiro Egi, Tomo-o Satoh, Kyohei Kanomata, Takashi Ikawa, and Shuji Akai*
Synlett, 2021, 32, 822–828.
DOI: 10.1055/a-1344-8713
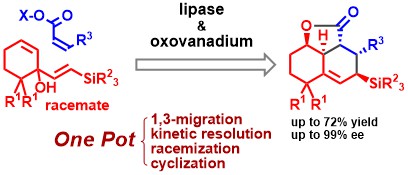
Abstract: Starting from readily available tertiary alcohols, four different reactions (a 1,3-migration of a hydroxy group, kinetic resolution, racemization, and an intramolecular Diels–Alder reaction) took place under co-catalysis by lipase and oxovanadium compounds in a one-pot process to produce polysubstituted tricyclic carbon frameworks in high yields and with high enantioselectivities. The key to the success of this process was the discovery that a silyl group attached to the terminal carbon of the vinyl moiety completely controls the direction of hydroxyl group migration
1) Regio-Complementary Preparation of 6- and 7-Fluoro-1,2,3,4-tetrahydroquinolines via the Cyclization of Catecholamines Followed by Deoxyfluorination
Kazuyuki Saito, Wang Zhou, Shohei Sato, Keita Takubo, Kazunori Furutsu, Ahmed A. B. Mohamed, Euis Maras Purwati, Takashi Ikawa, and Shuji Akai*
Heterocycles, 2021, 103, 902.
DOI: 10.3987/COM-20-S(K)56
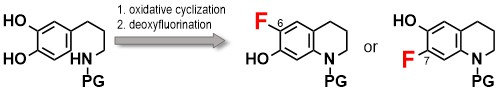
Abstract: We herein report a regioselective preparation of 6- and 7-fluoro-1,2,3,4-tetrahydroquinolines by applying the deoxyfluorination strategy, developed by the authors. This method includes the cyclization of catecholamines bearing an N-protecting group to form 7-hydroxy-1-azaspiro[4.5]deca-6,9-dien-8-ones and 6,7-dihydroxy-1,2,3,4-tetrahydroquinolines followed by deoxyfluorination, in which the nature of the N-protecting group has a significant effect on both the cyclization and the regioselectivity of the deoxyfluorination reaction.