9) Dynamic Kinetic Resolution for Asymmetric Synthesis: Synergy of Enzyme and Metal Catalysts,
Shuji Akai, Gamal A. I. Moustafa, “Kinetic Control in Synthesis and Self-Assembly” Ed. by Munenori Numata, Shiki Yagai, and Toshiyuki Hamura,
Academic Press, 2019, Chapter 2. ISBN: 9780128121269, Date book posted: 30 November 2018
Abstract: Developing an effective methodology for obtaining optically pure compounds at a quantitative yield has been the main concern in the field of organic chemistry. In this chapter, Akai and Moustafa review the concept of dynamic kinetic resolution as a potential solution for overcoming this fundamental issue. The advantage of the use of an enzyme (such as lipase) and its combination with metal catalysts is demonstrated in this chapter.
8) Microflow Fluorinations of Benzynes: Efficient Synthesis of Fluoroaromatic Compounds
Takashi Ikawa,* Shigeaki Masuda, Shuji Akai,
Chem. Pharm. Bull.,66, 1153 (2018).
DOI: 10.1248/cpb.c18-00578 Selected as Highlighted Article

Abstract: Fluorinated aromatic compounds are found in a variety of biologically active compounds, including clinical drugs and agrochemicals. Therefore, the synthesis of aryl fluorides is particularly important in the medicinal and process chemistry fields. In this paper, we report a method for the synthesis of aryl fluorides by benzyne fluorination under microflow conditions using the efficient Comet X-01 micromixer. In comparison to our previously reported method under ordinary batch conditions, this approach facilitates a significant reduction in reaction times, to ~10 s, as well as increases in the yields of fluoroarenes (by up to 51%).
7) Total synthesis of (-)-agelastatin A: an SH2’ radical azidation strategy
Izuru Tsuchimochi, Yuta Kitamura, Hiroshi Aoyama, Shuji Akai, Keiyo Nakai, Takehiko Yoshimitsu,
Chem. Commun., 54, 9893-9896 (2018).
DOI: 10.1039/c8cc05697h Cited as a cover picture
Abstract: A reagent generated from TMSN3/KMnO4/BnEt3NCl was found to promote an SH2’ radical azidation of a bromo silyl enol ether to furnish an azido silyl enol ether via olefin transposition. With the present azidation protocol, a new synthetic approach to agelastatin A, a potent antitumor marine alkaloid, has been established.
6) Flow Synthesis of (3R)- and (3S)-(E)-1-Iodohexa-1,5-dien-3-ol: Chiral Building Blocks for Natural Product Synthesis
Sota Katayama, Tomoyuki Koge, Satoko Katsuragi, Shuji Akai, Tohru Oishi Chem. Lett. 47, 1116-1118 (2018).
DOI:10.1246/cl.180475

Abstract: A concise procedure to prepare optically active (3R)- and (3S)-(E)-1-iodohexa-1,5-dien-3-ol was developed. The racemic alcohol was prepared under flow and batch conditions via successive half reduction of the ester followed by Grignard reaction. Lipase catalyzed kinetic resolution under flow conditions afforded (3S)- alcohol and corresponding (3R)-acetate. The acetyl group was removed under flow conditions by using ion exchange resin.
5) Lipase-Catalyzed Dynamic Kinetic Resolution of C1- and C2- Symmetric Racemic Axially Chiral 2,2′-Dihydroxy-1,1′-biaryls
Gamal A. I. Moustafa, Yasuhiro Oki, Shuji Akai,
Angew. Chem. Int. Ed., 57, 10278-10282 (2018),
DOI:10.1002/anie.201804161
Cited as a cover picture
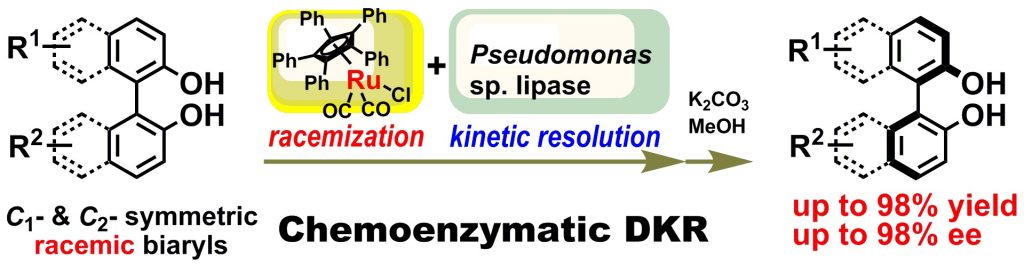

Abstract: We have discovered that the racemization of configurationally stable, axially chiral 2,2’-dihydroxy-1,1’-biaryls proceeds with a catalytic amount of a cyclopentadienylruthenium(II) complex at 35–50 °C. Combining this racemization procedure with lipase-catalyzed kinetic resolution led to the first lipase/metal-combo-catalyzed dynamic kinetic resolution of racemic axially chiral biaryls. The method was applied to the synthesis of various enantio-enriched C1– and C2-symmetric biaryl diols in yields of up to 98% and enantiomeric excesses of up to 98%, which paves the way for new developments in the field of asymmetric synthesis.
4) Lipase-catalyzed asymmetric synthesis of naphtho[2,3-c]furan-1(3H)-one derivatives by a one-pot dynamic kinetic resolution/intramolecular Diels–Alder reaction: Total synthesis of (–)-himbacine
Koji Sugiyama, Shinji Kawanishi, Yasuhiro Oki, Marin Kamiya, Ryosuke Hanada, Masahiro Egi, Shuji Akai,
Bioorg. Med. Chem., 26, 1378-1386 (2018),
DOI:10.1016/j.bmc.2017.08.019

Abstract: One-pot sequential reactions using the acyl moieties installed by enzymatic dynamic kinetic resolution of alcohols have been little investigated. In this work, the acryloyl moiety installed via the lipase/oxovanadium combo-catalyzed dynamic kinetic resolution of a racemic dienol [4-(cyclohex-1-en-1-yl)but-3-en-2-ol or 1-(cyclohex-1-en-1-yl)but-2-en-1-ol] with a (Z)-3-(phenylsulfonyl)acrylate underwent an intramolecular Diels–Alder reaction in a one-pot procedure to produce an optically active naphtho[2,3-c]furan-1(3H)-one derivative (98% ee). This method was successfully applied to the asymmetric total synthesis of (–)-himbacine.
3) オキソバナジウム/加水分解酵素混合触媒による第二級アルコールの動的光学分割:メソポーラスシリカの細孔の活用
Dynamic Kinetic Resolution of Secondary Alcohols by Oxovanadium/Hydrolase Combo-Catalysis: Effective Use of Mesoporous Silicas
赤井周司, ゼオライト, 35, 23-29 (2018); Shuji Akai, Zeolite, 35, 23-29 (2018)
Abstract: The dynamic kinetic resolution (DKR) has gained increasing attention because it is a simple method to obtain optically pure compounds from racemic substrates in quantitative yields. DKR is typically performed by a combination of kinetic resolution of racemates and in situ, continuous, and rapid interconversion between two enantiomers of the substrates, namely, racemization. We recently reported a new DKR method based on the combination of lipase-catalyzed kinetic resolution of racemic secondary alcohols and the V-MPS-catalyzed in situ racemization. In V-MPS, oxovanadium moieties were covalently bound to the inner surface of mesoporous silica (MPS) with a pore size of 2–4 nm. In this article, we introduce the background of why we used MPS, reaction characteristics of V-MPS, advantages of our DKR method, and its synthetic applications.
2) 3-(Triflyloxy)benzynes Enable the Regiocontrolled Cycloaddition of Cyclic Ureas to Synthesize 1,4-Benzodiazepine Derivatives
Hideki Kaneko, Takashi Ikawa, Yuta Yamamoto, Sundaram Arulmozhiraja, Hiroaki Tokiwa, Shuji Akai,
Synlett, 29, 943-948 (2018), DOI:10.1055/s-0036-1591924

Abstract: A versatile synthesis of 1,4-benzodiazepine derivatives through the reaction of various 3-(trifluoromethanesulfonyloxy)benzynes with N-(p-toluenesulfonyl)imidazolidin-2-ones is reported. This reaction system provides a 1,4-benzodiazepine bearing a trifluoromethanesulfonyloxy group as a single regioisomer among the four possible regioisomers.
1) Synthesis of Optically Active 2,3-Disubstituted Indoline Derivatives through Cycloaddition Reactions between Benzynes and α,β-Unsaturated γ-Aminobutyronitriles
Takashi Ikawa, Yuta Sumii, Shigeaki Masuda, Ding Wang, Yuto Emi, Akira Takagi, Shuji Akai,
Synlett, 29, 530–536 (2018),
DOI:10.1055/s-0036-1591722

Abstract: We report a method for synthesizing optically active 2,3-disubstituted indolines by the cycloaddition reaction of benzynes with various 4-[(4-toluenesulfonyl)amino]-(E)-but-2-enenitriles, which are readily prepared from the corresponding ʟ-amino acid derivatives.