11) Regioselective Rearrangement of 4,4-Disubstituted 2-Hydroxycyclohexa-2,5-dienones under Deoxyfluorination Conditions
Keita Takubo, Ahmed A.B. Mohamed, Takafumi Ide, Kazuyuki Saito, Takashi Ikawa, Takehiko Yoshimitsu, Shuji Akai, J. Org. Chem., 82, 13141-13151 (2017), DOI:10.1021/acs.joc.7b02208

Abstract: The dienone-phenol rearrangement is a useful tool for the synthesis of highly substituted phenols. In our previous study of the rearrangement of 4,4-disubstituted 2-hydroxycyclohexa-2,5-dienone under deoxyfluorination conditions, bond migration proceeded with very poor regioselectivity. In this paper, an acid-mediated rearrangement of O-perfluoroalkylsulfonyl difluorides with regioselective migration toward the β’-carbon is reported. This method allowed the synthesis of a fluorinated analog of allocolchicinoids with improved total yield. Successful application to other substrates was also demonstrated.
10) Hydrolase-Catalyzed Dynamic Kinetic Resolution of Racemic Alcohols
Shuji Akai, “Future Directions in Biocatalysis, 2nd Edition”. Ed. by Tomoko Matsuda, Elsevier, 2017, Chapter 16., ISBN: 978-0-444-63743-7, Date book posted: 21 September 2017
Abstract: This chapter introduces the recent aspects on the hydrolase-catalyzed dynamic kinetic resolution of racemic alcohols, which is made of the following sections and has 97 references.
16.1 Introduction and General Aspects
16.2 Dynamic Kinetic Resolution With Redox Catalysts
16.3 Dynamic Kinetic Resolution With Oxometal Catalysts
16.4 Dynamic Kinetic Resolution With Acid or Base Catalysts
16.5 Concluding Remarks and Future Perspectives
9) Unified, Efficient, and Scalable Synthesis of Halichondrins: Zirconium/Nickel-Mediated One-Pot Ketone Synthesis as the Final Coupling Reaction
Kenzo Yahata, Ning Ye, Yanran Ai, Kentaro Iso, Yoshito Kishi, Angew. Chem., Int. Ed., 56, 10796-10800 (2017), DOI:10.1002/anie.201705523
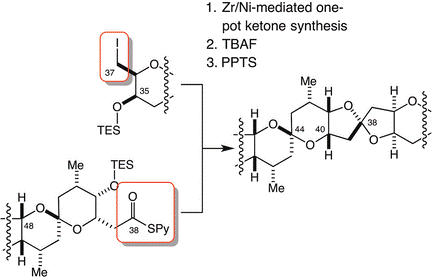
Abstract: The halichondrin natural products were obtained by unified, efficient, and scalable total syntheses. A newly developed zirconium/nickel-mediated one-pot ketone synthesis was used to couple the two halves of the final product at a late stage of the total synthesis.
8) Zirconium/Nickel-Mediated One-Pot Ketone Synthesis
Yanran Ai, Ning Ye, Qiaoyi Wang, Kenzo Yahata, Yoshito Kishi, Angew. Chem., Int. Ed., 56, 10791-10795 (2017), DOI:10.1002/anie.201705520
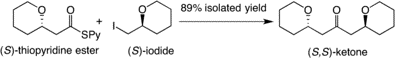
Abstract: A zirconium/nickel-mediated one-pot synthesis of ketones is reported. In the presence of metallic zinc or manganese, Cp2ZrCl2 dramatically accelerates the coupling and suppresses side product formation. This method is applicable to nucleophiles bearing OR or equivalent functional groups at the α-position.
7) Stereocontrolled Synthesis of Left Halves of Halichondrins
Kenzo Yahata, Ning Ye, Kentaro Iso, Yanran Ai, Jihoon Lee, Yoshito Kishi, J. Org. Chem., 82, 8808-8830 (2017), DOI:10.1021/acs.joc.7b01284
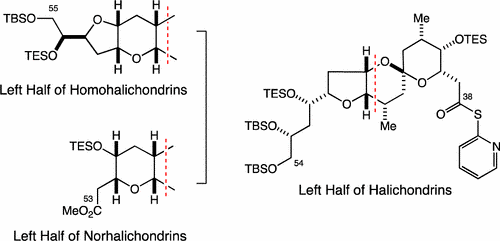
Abstract: A stereocontrolled synthesis of the left halves of halichondrins was reported. An intramolecular oxy-Michael reaction under basic conditions was used to construct the [6,6]-spiroketal in a stereocontrolled manner. With this approach, the left halves of halichondrins, homohalichondrins, and norhalichondrins were synthesized.
6) Unified Synthesis of Right Halves of Halichondrins A-C
Kenzo Yahata, Ning Ye, Kentaro Iso, Santhosh Reddy Naini, Shuji Yamashita, Yanran Ai, Yoshito Kishi, J. Org. Chem., 82, 8792-8807 (2017), DOI:10.1021/acs.joc.7b01283
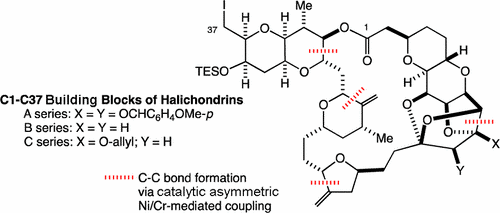
Abstract: The right halves of halichondrins A–C were synthesized by coupling the common C20–C37 building block with the C1–C19 building blocks, respectively. Catalytic, asymmetric Ni/Cr-mediated coupling was used for three C–C bond formations. For all cases, the stereochemistry was controlled with the Cr catalyst prepared from the chiral sulfonamide identified via the toolbox approach. For each couplings, the stereoselectivity of 28:1, >40:1, and ∼20:1 was achieved by the Cr catalysts. Unlike the first and second couplings, the third coupling used the structurally complex nucleophile. It was demonstrated that the coupling efficiency was excellent even with the electrophile/nucleophile molar ratio = 1.0/1.1. In addition, the third coupling was achieved with the substrate bearing a free hydroxyl group. The products obtained in the Ni/Cr-mediated couplings were converted to the right halves of halichondrins A–C in excellent overall yields. The right halves of halichondrins A–C were synthesized in 28, 24, and 24 steps from commercial D-galactal in 13.4%, 21.1%, and 16.7% overall yield, respectively.
5) 加水分解酵素と金属の触媒集積型動的光学分割:ラセミ体アルコールを光学的に純粋な化合物に収率100%で変換する新手法
Dynamic Kinetic Resolution by Hydrolase–Metal Integrated Catalysis: A Novel Method for the Quantitative Conversion of Racemic Alcohols into Optically Pure Compounds
赤井周司, 有合化誌, 75, 441-448 (2017), Shuji Akai, Synth. Org. Chem. Japan, 75, 441-448 (2017), DOI:10.5059/yukigoseikyokaishi.75.441
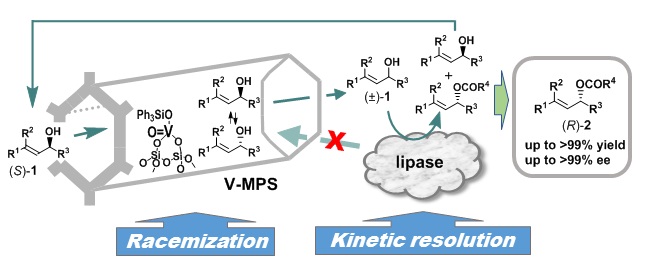
Abstract: Hydrolase-catalyzed dynamic kinetic resolution (DKR) of racemic secondary alcohols has made a significant progress in the last two decades, and this process has become one of the most convenient and practical methods to convert racemic alcohols into enantiopure compounds in up to 100% yields. Such DKR is performed using an integration of the hydrolase-catalyzed kinetic resolution of racemic alcohols and the in-situ racemization of the remaining less reactive enantiomers by other catalysts, majority of which include metallic redox catalysts and oxovanadium compounds. This article mainly introduces the DKR of secondary alcohols by hydrolase–oxovanadium integrated catalysis developed by the author’s group. Some examples of their applications to asymmetric synthesis of natural products are also discussed.
4) Fe/Cu-Mediated One-Pot Ketone Synthesis
Vemula Praveed Kumar, Vaddela Sudheer Babu, Kenzo Yahata, Yoshito Kishi, Org. Lett., 19, 2766-2769 (2017), DOI:10.1021/acs.orglett.7b01128
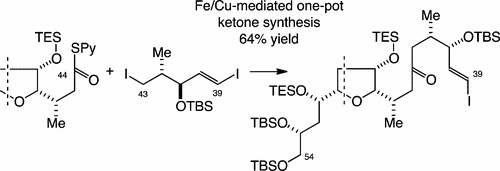
Abstract: An Fe/Cu-mediated one-pot ketone synthesis was reported. Unlike Ni- and Pd-mediated one-pot ketone syntheses, the reported Fe/Cu-mediated method allowed selective activation and coupling of alkyl iodides over vinyl iodides. The newly developed one-pot ketone synthesis was applied to a synthesis of vinyl iodide/ketone, the left half of halichondrin B, as well as vinyl iodide/ketone, the C20–C26 building block of halichondrins.
3) 2-(Trimethylsilyl)phenyl Trimethylsilyl Ethers as Stable and Readily Accessible Benzyne Precursors
Takashi Ikawa, Shigeaki Masuda, Hiroki Nakajima, Shuji Akai, J. Org. Chem., 82, 4242-4253 (2017), DOI:10.1021/acs.joc.7b00238

Abstract: Stable 2-(trimethylsilyl)phenyl trimethylsilyl ethers, readily obtained from the corresponding halogenated phenols in two steps, were identified as novel benzyne precursors. These species were converted to benzynes by a domino reaction of O-desilylation, O-nonaflylation, and β-elimination under mild conditions using nonafluorobutanesulfonyl fluoride (NfF) and tetrabutylammonium triphenyldifluorosilicate (TBAT). The generated benzynes were trapped by various arynophiles to afford a wide variety of benzo-fused heterocycles.
2) MPP+ induces necrostatin-1- and ferrostatin-1-sensitive necrotic death of neuronal SH-SY5Y cells
Keisuke Ito, Yutaka Eguchi, Yusuke Imagawa, Shuji Akai, Hideki Mochizuki, Yoshihide Tsujimoto, Cell Death Discovery, 3, 17013 (2017), DOI:10.1038/cddiscovery.2017.13
Abstract: Regulation of cell death is potentially a powerful treatment modality for intractable diseases such as neurodegenerative diseases.Although there have been many reports about the possible involvement of various types of cell death in neurodegenerative diseases, it is still unclear exactly how neurons die in patients with these diseases, thus treatment strategies based on cell death regulation have not been established yet. To obtain some insight into the mechanisms of cell death involved in neurodegenerative diseases, we studied the effect of 1-methyl-4-phenylpyridinium (MPP+) on the human neuroblastoma cell line SH-SY5Y (a widely used model of Parkinson’s disease). We found that MPP+ predominantly induced non-apoptotic death of neuronally differentiated SH-SY5Y cells. This cell death was strongly inhibited by necrostatin-1 (Nec-1), a necroptosis inhibitor, and by an indole-containing compound (3,3′-diindolylmethane: DIM). However, it occurred independently of receptor-interacting serine/threonine-protein kinase 1/3 (RIP1/RIP3), indicating that this form of cell death was not necroptosis. MPP+-induced cell death was also inhibited by several inhibitors of ferroptosis, including ferrostatin-1 (Fer-1). Although MPP+-induced death and ferroptosis shared some features, such as occurrence of lipid peroxidation and inhibition by Fer-1, MPP+-induced death seemed to be distinct from ferroptosis because MPP+-induced death (but not ferroptosis) was inhibited by Nec-1, was independent of p53, and was accompanied by ATP depletion and mitochondrial swelling. Further investigation of MPP+-induced non-apoptotic cell death may be useful for understanding the mechanisms of neuronal loss and for treatment of neurodegenerative diseases such as Parkinson’s disease.
1) Preparation of optically active cycloalkenes bearing all-carbon quaternary stereogenic centres via lipase–oxovanadium combo-catalysed dynamic kinetic resolution
Shinji Kawanishi, Koji Sugiyama, Yasuhiro Oki, Takashi Ikawa, Shuji Akai, Green Chem., 19, 411-417 (2017), DOI:10.1039/C6GC01995A
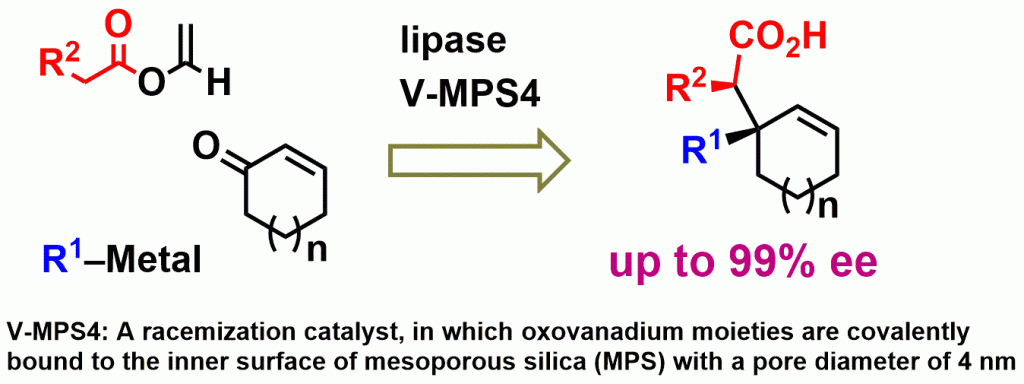
Abstract: In this study, a novel asymmetric synthesis of all-carbon quaternary stereogenic centres is developed by the connection of three prochiral or achiral components–conjugated enones, organometallic compounds and vinyl esters–at the C-1 position of the enones. This method involves three sequential steps: 1,2-nucleophilic addition of an organometallic compound to the enone, lipase-catalysed dynamic kinetic resolution (DKR) of the tert-allylic alcohol and the Ireland-Claisen rearrangement of the optically active allyl ester thus generated. This method features the effective use of acyl moieties installed by DKR for achieving high atom economy. The application of this method to the protective-group-free asymmetric total synthesis of (-)-crinane, a core structure of a class of natural alkaloids, demonstrated that it can alter a known synthetic pathway of a racemate into an asymmetric synthesis of an optically pure molecule while reducing the total transformation steps and increasing the overall yield. With these advantages, this method is practical and attractive as a new environmentally benign protocol.